Professor Jon Cooper
Our activities in synthetic biology involve using microfluidics to create cell chassis comprising polymerosomes or lipid bilayers. Using cell-free expression systems we have already shown the assembly of functional proteins at such membranes, within artificial cells. We have now designed new transporter proteins which are being expressed with the prospect of assembling them to display extracellular proteins on the external surface of the artificial cells. This paradigm will enable us to control the structure-geometry of synthetic biological assemblies, created with expressed ligand-binding motifs to tailor the chassis' functionality. The low-cost desktop biomanufacturing unit can create 1 mm3 of material/second of high value materials that can be used in biochemical engineering, protein purification as well as tissue and cell engineering (by co-seeding with stem cells, where the display protein is a cell-cue).
Synthetic biology is an emerging field of science that offers the prospect of the design and construction of new biological pathways and or systems that do not exist in nature. Using a “bottom-up approach” of assembling components, such as biochemical pathways, it has the potential to exploit our biological understanding in much the same way that circuit design enabled electronics. We are working on five major interrelated challenges in my labs.
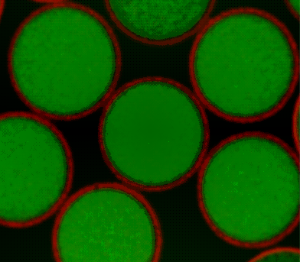
Fig. 1 Artificial cell chassis (or proto-cells), defined by a lipophilic membrane (red), inside which a protein has been expressed from DNA (green). Size = 20μm.
• Assembling components for the expression and coordination of multiple genes. We develop synthetic recombinase systems for use in the rapid generation, evolution and optimisation of gene circuits and metabolic pathways. These techniques have also been used in our labs to assemble molecular constructs on surfaces using microarray technologies.
• Creating artificial cell surrogates, or protocells, which enable biochemical pathways to be reconstructed, to perform complex processing steps. We have already expressed membrane associated proteins in such protocells and watched them assemble in the artificial cells using time-resolved techniques.
• Understanding the signalling mechanisms that control cell (or proto-cells) behaviours, using advanced modelling techniques. The analogy between cell signalling and complex control systems or indeed communication networks is one that has helped engineers to engage with biologists to understand the nature of cell signaling and disease.
• Using integrated microfluidic devices to address several of the issues that currently hinder the rational design of gene circuits. We are interested in developing this platform for parallel, high-throughput in vitro assays, and the idea that by miniaturising laboratory functions microfluidics could permit large-scale 'prototyping' of synthetic constructs.
• Developing low cost diagnostics for bacteria sensing in water and healthcare for use in resource-poor environments.
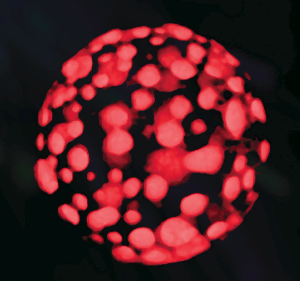
Fig. 2 Self-organising domains on an artificial cell membrane.

