Virulence mismatches in hosts shape outcomes of cross-species transmission
Published: 17 November 2020
A new study involving Centre for Virus Research scientists has found cross-species disease progression is accelerated, which reduced the chances of onwards transmission, when the original and new hosts were physiologically or genetically dissimilar.

A new study involving Centre for Virus Research scientists has found cross-species disease progression is accelerated, which reduced the chances of onwards transmission, when the original and new hosts were physiologically or genetically dissimilar.
Infectious disease emergence is often the result of a pathogen entering a new host species, as highlighted by COVID-19. However, most cross-species transmissions fail to establish in the newly-infected species.
For diseases to emerge, pathogens not only need to infect a novel host but also be subsequently transmitted from one individual to another – a critical step. Why some pathogens succeed at this point while others fail is not well understood.
In this study, authored by Dr Nardus Mollentze, Dr Daniel Streicker, Professor Pablo Murcia, Professor Katie Hampson (IBAHCM), and Dr Roman Biek (IBAHCM) and published in PNAS, researchers analysed hundreds of published infection experiments involving the transfer of rabies virus from one host species to another.
The study found that rabies virus strains originating in bats – and those transferred from species with warmer body temperatures to those with a cooler body temperature – tended to kill the first infected host too fast for successful onward transmission.
The same was true as the genetic distance between the original and infected host species increased.
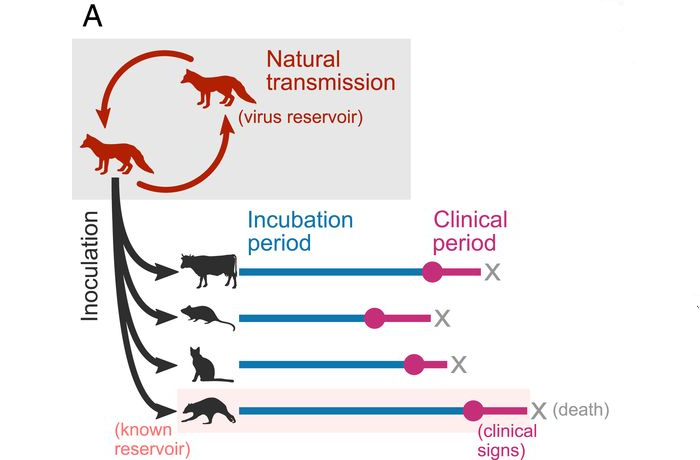
Fig 1a
Dr Nardus Mollentze, Postdoctoral Research Assistant at the CVR, said: “Our results indicate that rabies virus strains are finely adapted to a specific host environment, and that even subtle mismatches between virus and host can limit the virus’ ability to establish transmission in a novel species.
“These findings help to explain why rabies virus is maintained by a rather small subset of mammalian hosts and why emergence in new species is observed only in rare cases.”
Dr Roman Biek, Reader at the Institute of Biodiversity, Animal Health and Comparative Medicine, said: “Our work shows how careful analysis of previous infection experiments can pinpoint small changes in the interaction between pathogen and host that may either help or hinder disease emergence, including emergence of new human infectious diseases.
“This is why investigating the factors that affect the clinical outcomes of cross-species transmission in the first novel host individual infected, the index host, is important, as these outcomes can alter the likelihood of the pathogen spreading further. “
Dr Mollentze added: “As cross-species transmission events are very difficult to observe in nature, we currently remain unable to predict which of them could potentially result in a new epidemic.
“Studies like ours demonstrate that already-existing experimental infection data can be used to better understand cross-species infections, which, if applied to more viruses, may allow us to identify general rules predicting onward spread in the future.”

Virulence mismatches in index hosts shape the outcomes of cross-species transmission
- Nardus Mollentze, Daniel G. Streicker, Pablo R. Murcia, Katie Hampson, and Roman Biek.
- PNAS, first published October 29, 2020; https://doi.org/10.1073/pnas.2006778117
- The work was funded by the Medical Research Council, Wellcome, the Royal Society, and a Lord Kelvin/Adam Smith Scholarship from the University of Glasgow.
Image (Fig 1a): Data were collected from published experiments in which naturally circulating rabies virus isolates were inoculated into heterologous species. The delay between inoculation and the appearance of clinical signs (the incubation period) and the delay between clinical signs first appearing and death (the clinical period) was recorded for individual animals.
Enquiries: ali.howard@glasgow.ac.uk or elizabeth.mcmeekin@glasgow.ac.uk / 0141 330 6557 or 0141 330 4831
First published: 17 November 2020

