Research
Our main research interest is the synthesis of biologically active natural products, such as taxol, a powerful antitumor agent, or dolabelide C and hexacyclinic acid, molecules with cytotoxic activity. In our approaches towards the target molecules, we uncover problems that cannot be easily solved by known reactions, and to overcome these difficulties we try to develop new methodologies that can also be applied to other syntheses.
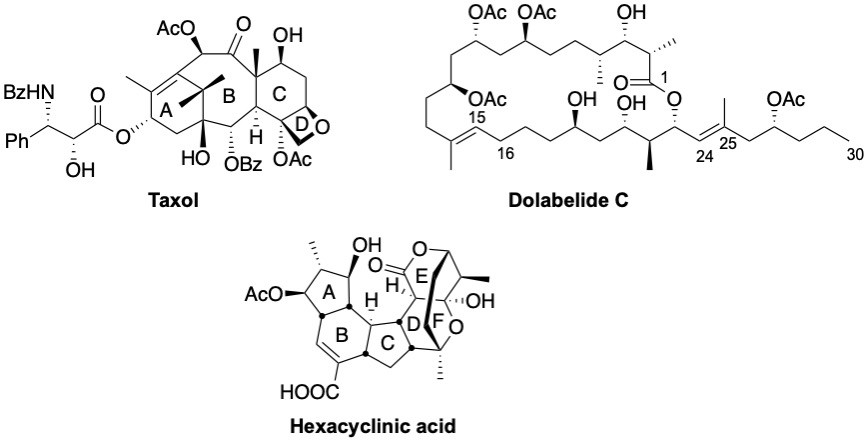
Taxol
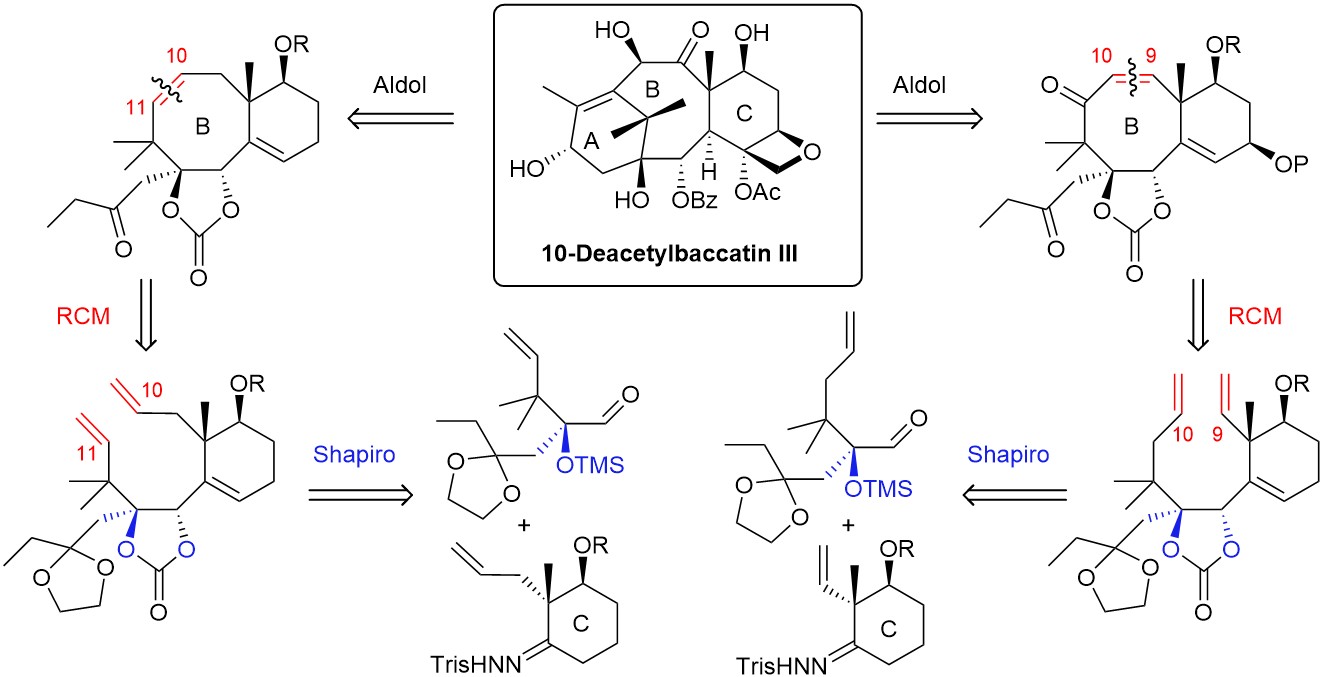
We envisioned two retrosyntheses for the synthesis 10-deacetylbaccatin III, which is a natural precursor of taxol. They involve a ring-closing metathesis (RCM) to close the eight-membered B ring between C9 and C10 or C10 and C11, and a Shapiro coupling to synthesize the RCM precursors. These two approaches have enabled us to synthesize model BC ring systems of taxoids, and we are exploiting the C10-C11 metathesis route for the synthesis of 10-deacetylbaccatin III.
Dolabelide C
During the synthesis of Dolabelide C, we have developed new methodologies for the construction of protected allylic syn 1,3-diols. We have reported a diastereoselective intramolecular conjugate addition of a hemiacetal anion, made in situ from a homoallylic alcohol and benzaldehyde in the presence of base, to a vinyl sulfone. The benzylidene acetal was reduced to free the proximal hydroxy group, and addition of the dianion of the β-hydroxy sulfones to aldehydes was successful, but an additional two steps (acetate or benzoate formation followed by reductive elimination) is required to form the alkene, with the issue of differentiating the two hydroxy groups during acylation.
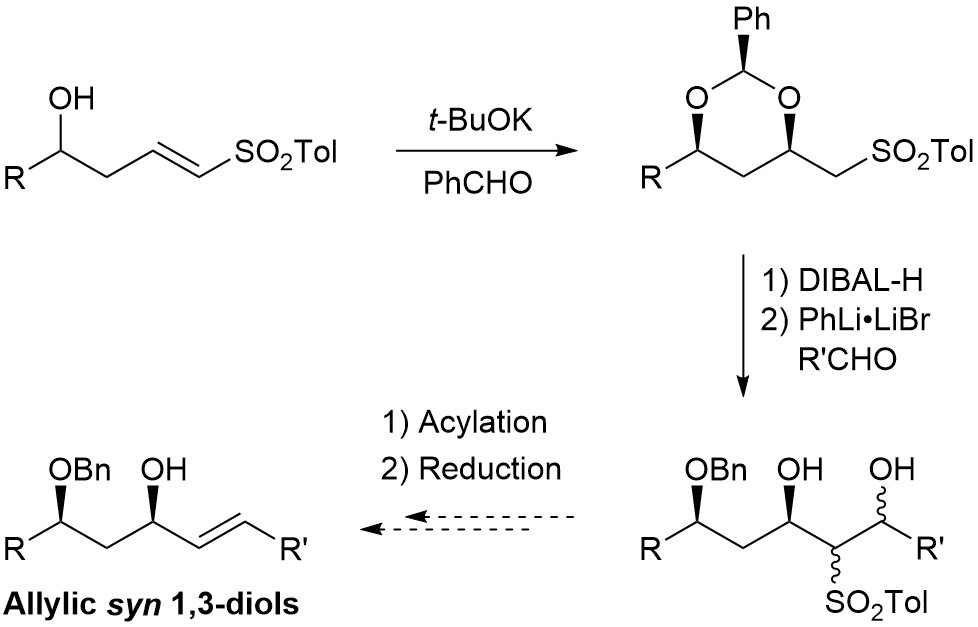
We thus developed a new sequence to reach these allylic syn 1,3-diols, where the condensation reaction with the aldehydes took place before the oxa-Michael step. An oxa-Michael/acetate elimination cascade, followed by reduction of the resulting sulfone, gave the desired allylic syn 1,3-diols with high diastereoselectivity and good E/Z ratio. This conjugate addition/elimination sequence was also successful with esters as Michael acceptors, to give functionalized Morita-Baylis-Hillman adducts.
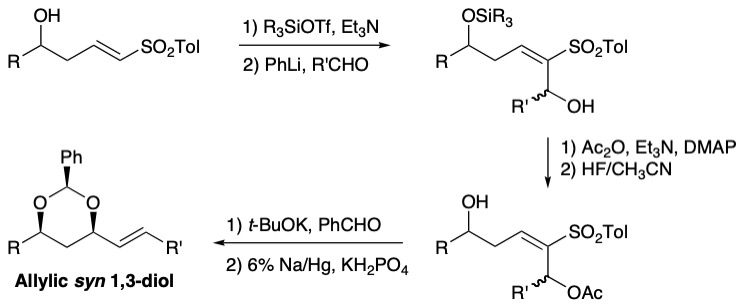
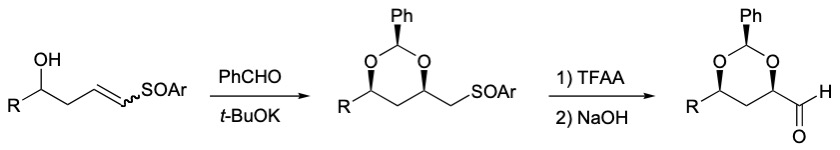
More recently, we have succeeded in synthesizing these protected allylic syn 1,3-diols in two steps from hydroxy vinyl heteroaromatic sulfones.
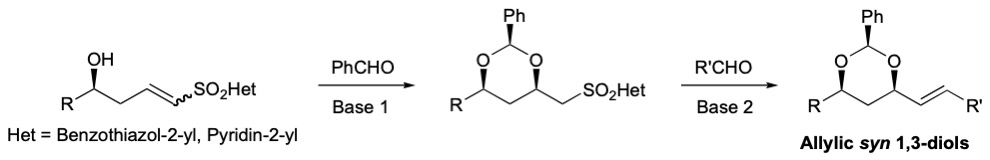
The same type of conjugate addition has also been described by us with carbamate sulfones as Michael acceptors. The resulting benzylidene acetals were transformed in two steps to synthons encompassing an aldehyde a to the dioxane ring, which were further transformed into protected allylic syn 1,3-diols by a Wittig olefination.
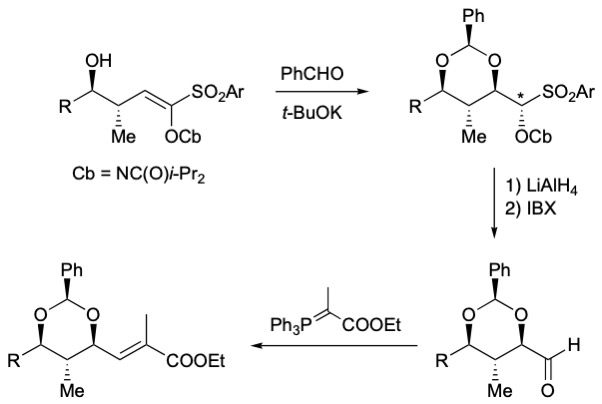
The above aldehydes can also be synthesized by a Pummerer rearrangement from the corresponding sulfoxides. These sulfoxides were prepared by a diastereoselective oxa-Michael reaction from hydroxy vinyl sulfoxides.
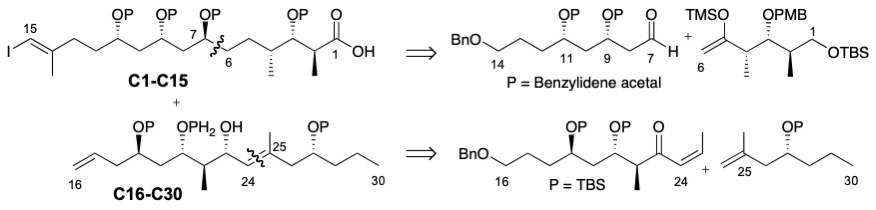
For the synthesis of dolabelide C, we have reported the construction of the C1-C15 fragment, which features a diastereoselective Mukaiyama aldol as the key step. The protected syn 1,3-diol at C9 and C11 was installed by an oxa-Michael reaction on the unsaturated ester. The C16-C24 fragment was synthesized by applying the new methodologies mentioned above. Construction of the C16-C30 portion is underway, featuring a highly hindered olefin cross-metathesis to build the C24-C25 bond.
Hexacyclinic acid
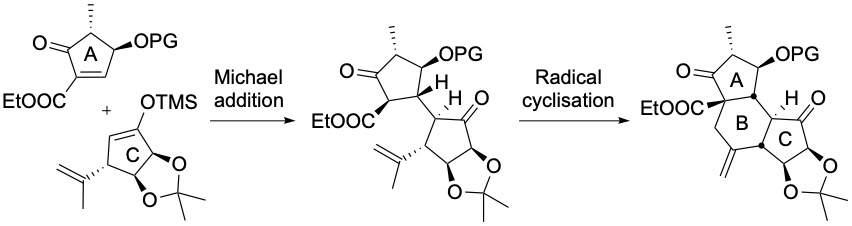
Finally, we are exploring the synthesis of hexacyclinic acid. The ABC tricycle has been synthesized using a highly diastereoselective Michael addition and a radical cyclization as key steps. The construction of the DEF tricycle is in progress.
Post-polymerisation functionalisation by olefin metathesis
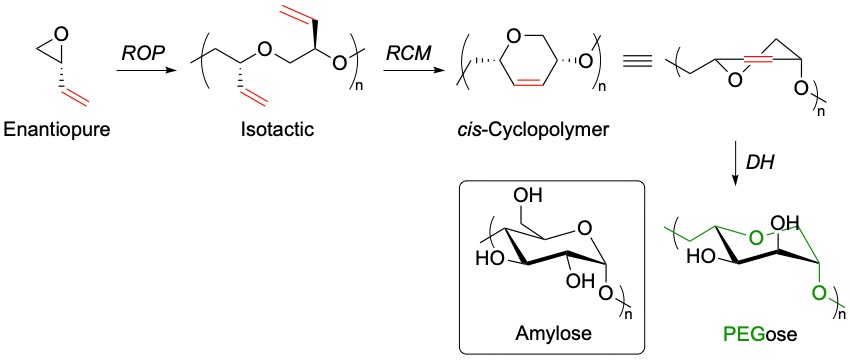
The Prunet group has also been using metathesis reactions for the post-polymerisation functionalisation of a diverse array of polymers with pendent olefin groups. In collaboration with Prof. Christophe Thomas (Chimie ParisTech), the CM of polyesters and diverse small molecule partners was used to afford functionalised polymers with a dramatic increase in their glass-transition temperature compared to the starting polyesters. With Prof. Rob Liskamp (University of Glasgow), three novel polyethers with differently substituted olefinic side chains were prepared, tailored to promote cross metathesis (CM) vs self metathesis (SM), and the first successful CM between a polymer and a coupling partner of biological relevance, the tripeptide RGD, was performed. Insoluble beads of poly(divinylbenzene) prepared in the group of Prof. Peter Cormack (University of Strathclyde) could be functionalised by CM to provide ion-exchange resin materials. Finally, in collaboration with Porf. Michael Shaver (University of Manchester), RCM of isotactic polyepoxybutene (PEB) was used to prepare a stereocontrolled 1,4-linked cis cyclopolyether. Post-polymerisation dihydroxylation (DH) then furnished a poly(ethylene glycol) backbone with sugar-like functionalities (PEGose), which mimics the structure of amylose.