Non laser fluorescence microscope optical equipment
ZEISS VIVATOME APERTURE CORRELATION MOTORISED INVERT MICROSCOPE CONFOCAL AND EPIFLUORESCENCE IMAGING AVAILABLE..
Aperture Correlation fluorescence microscopy combines the light efficiency of structured illumination with the acquisition speed of a spinning disk confocal instrument and allows you to overcome the limited light efficiency encountered with traditional spinning disk confocal systems where a multi-pinhole array is used to sweep across the specimen.
The bottom port of our invert Zeiss Axio Observer microscope has been fitted with an aperture correlation VivaTome spinning disk unit. The disk is made of synthetic quartz which has been coated with a thin layer of aluminium which is arranged in a structured illumination pattern so that light is either efficiently reflected from the disk surface or passes through it.
An overview of the capabilities of this system can be found by clicking this web link:
ZEISS Microscopy Online Campus | Introduction to Aperture Correlation Microscopy (fsu.edu)
Instead of individual pinholes, the VivaTome uses a spinning structured illumination grid pattern to simultaneously image emission light that is reflected (R pathway) or transmitted (T pathway) through the spinning disk side by side on to the sensor of the camera detector. The side by side raw image is split to form 2 individual channel images (T) and (R). R image is mirrored (horizontally flipped) and then overlayed with the T channel image. The pixels in each channel image are co-registered with zero pixel shift. The overlay image is then split into 2 individual channel images, Treg and Rreg. The subtraction of these 2 images from one another (using a K weighting factor of 1 applied to the Rreg image) will form a confocal image whereas addition will generate a widefield image.
You can manipulate the K weighting factor (0-1) to alter the mix amount of confocal and widefield information present in the final image formed.
The microscope also has X, Y and Z motorised capability so various image recording modes can be easily configured (e.g. single or multichannel epifluorescence time lapse imaging in 2D or z stack 3D multifield imaging. The microscope also has Definite focus fitted to compensate for thermally induced z focus drift which can be problematic when recording time lapse images over lengthy time periods.
For very low fluorescence light emission detection, a Photometrics Evolve 512 EMCCD camera has been fitted to the left hand side microscope port for FRET, BRET & multi-epifluorescence imaging. Colibri LED's which allow µsec excitation light switching (µsecs) are used to minimise photobleaching when performing conventional multi-fluorescence or FRET experiments where large image data sets are TTL trigger streamed via a SVB1 trigger breakout box directly on to the hard drive every 5-30 msecs with precise precision. EMCCD camera has been fitted to an image splitting device to record Donor and FRET channel images simultaneously. Image splitter can also be used to record BRET. Acquisition software used to record images is Axiovision 4.8.2.
Objective Lens:
10x Plan-Neofluor, NA = 0.30, RI = air, WD = 5mm
20x LD Plan-Neofluor, NA = 0.40, RI = air, WD = 7.9 mm
40x Plan-Apochromat, NA = 1.40, RI =oil, DIC, WD = 0.13 mm
63x Plan-Apochromat, NA = 1.40, RI =oil, DIC, WD = 0.19 mm
Microscope turret fluorescence filter sets:
DAPI/HOECHST (Semrock LF405-A) set:
Excitation: 390/40nm;
Dichroic: Reflection = 372-410 nm/Transmission = 420.3 - 900 nm
Emission: 452/45 nm
ECFP (Chroma ET series 49001) set:
Excitation: 436/20 nm
Dichroic: T455 LP
Emission: 480/40 nm
EGFP (Chroma ET series 49002) set:
Excitation: 470/40 nm
Dichroic: T495 LP
Emission: 525/50 nm
EYFP (Chroma ET series 49003) set:
Excitation: 500/20 nm
Dichroic: T515 LP
Emission: 535/30 nm
mCherry/TRITC/Tx Red/Rhodamine (Chroma ET series 49008) set:
Excitation: 560/40 nm
Dichroic: T585 LP
Emission: 630/75 nm
VivaTome Spinning Disk filter sets (Semrock):
Set 1: Excitation = 406/15 nm; Emission = 457/50 nm
Set 2: Excitation = 494/20 nm; Emission = 536/40 nm
Set 3: Excitation = 575/20 nm; Emission = 628/40 nm
The rig is located in room 162 of the ARC Building on Level 1 and the contact person to book the system is Dr John Pediani; email address: John.Pediani@glasgow.ac.uk.
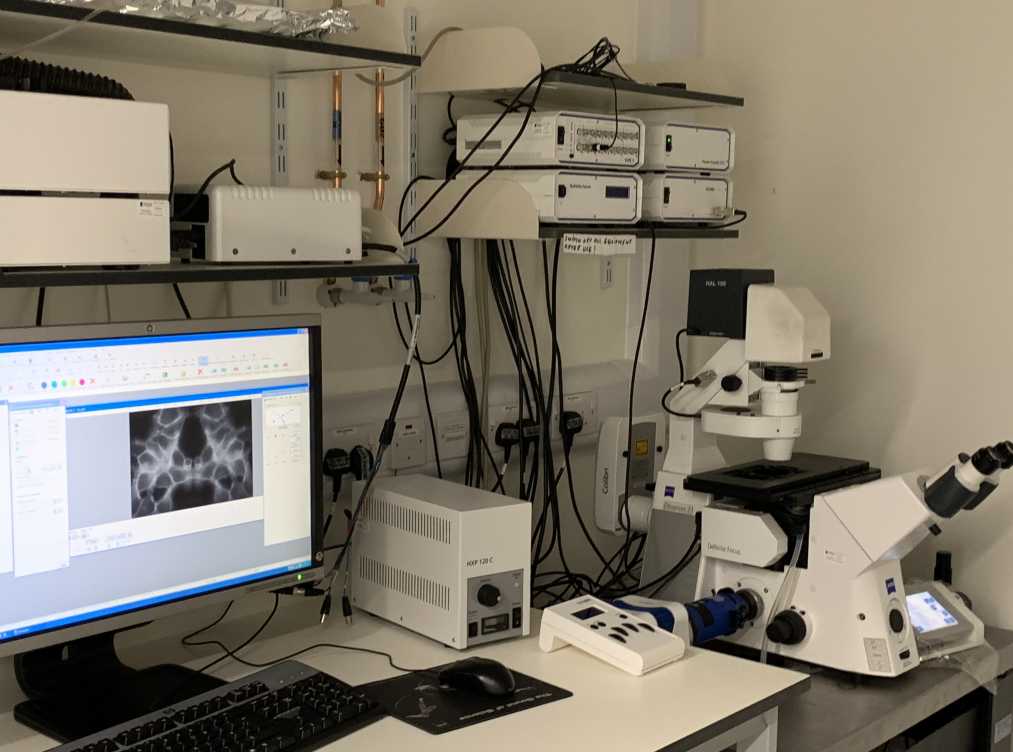
A picture of the Vivatome spinning disk microscope with Colibri LED illumination is illustrated below:
NIKON ECLIPSE TE 2000-E EPIFLUORESCENCE IMAGING MOTORISED MICROSCOPE.
NIKON ECLIPSE TE 2000-E MICROSCOPE.
This invert configured microscope rig has motorised Z control and can be operated in multiple configurations which allows users to perform single or multi epifluorescence experiments at high speed. Excitation fluorescence over the wavelength range from 320 to 650 nm is rapidly generated from a Cairn OptoScan monochromator and a 10 position emission filter wheel fitted to the left hand side port of the microscope is used for optimal detection of blue, cyan, green, yellow and red monomeric fluorescent proteins or fluorochromes. Photobleaching of samples is minimised by rapidly shuttering ( - 1 msec) the output slit of the monochromator. Emitted fluorescence signals are sequentially detected using a high resolution Hamamatsu ORCA FLASH 4.0 LT+ CMOS sensor camera coupled to an emission filter wheel. Alternatively, fluorescence emissions can be detected simultaneously with no time delay using an ORCA FLASH 4.0 LT+ CMOS camera coupled to a Quadview 2 image splitting device (Mag Biosystems) situated on the microscope's bottom port. The microscope has a built in linear z encoder for high precision acquisition of multi-fluorescence z stacks over time (4D). Rapid exchange of test ligands to cells grown on round glass coverslips is achieved by using an open cell perfusion chamber coupled to 2 solenoid valves via a 4-channel peristaltic pump. Acquisition software used to record multichannel images overtime is Metamorph version 7.8.13 and time lapse ratiometric imaging (e.g. Fura 8 calcium or REDOX potential imaging) can be performed using Metafluor version 7.8.13.
Objective Lens:
40x LWD Plan-Fluor, NA = 0.85, RI = air, WD = 2.7-3.7 mm
60x Plan Fluor, NA = 0.85, RI = air, WD = 0.30 mm
40x Superfluor, NA = 1.30, RI = oil, WD = 0.22mm
40x Plan-Apochromat, NA = 1.30, RI = oil, WD = 0.20
60x Plan-Apochromat, NA: 1.40, RI = oil, WD = 0.21 mm
Turret fluorescence filter set positions:
Position 1: CHROMA ET series sputter coated:
Fura2/QAPB/mEGFP/HOECHST; Ex=340-490; BS=505 DRLP-ET series, Em=515LP.
Position 2: CHROMA HQ: TRITC/Rhodamine FILTER SET (41002b); Ex=HQ545/30m, D=Q570 LP; Em=HQ610/75LP.
Position 3: CHROMA HQ, DAPI/HOECHST. Filter set C 68609:Dichroic is 420 DCLP; Emitter is HQ 450/50
Position 4 = Cyan-Yellow FRET (SEMROCK sputter coated). Donor Ex=427/10, Donor Em=472/30M; Acceptor Ex=504/12, Acceptor Em=542/27M; FRET Ex=427/10, FRET Em=542/27M
Position 5 = ECFP (31044v2bs), Ex=436/20, D=455DCLP, Em=480/40MHQ (excludes EYFP but not EGFP).
Position 6 = EYFP (41028v2bs), Ex=500/20, D=Q515LP, Em=535/30MHQ (excludes ECFP but not EGFP).
The microscope rig is located in room 162 of the ARC building on level 1 and the contact person to use the system is John Pediani, email address: john.pediani@glasgow.ac.uk. A picture of the Nikon microscope imaging system is illustrated directly below.

NIKON ECLIPSE TI-S EPIFLUORESCENCE IMAGE SCREENING MICROSCOPE.
NIKON ECLIPSE TI-S MICROSCOPE RIG:
This invert microscope has an OSRAM HBO 103/W2 Mercury lamp light source and a varietry of filter sets to view the fluorophores DAPI/HOECHST, ECFP, EGFP, EYFP and mCherry. A monochrome digital camera (DS Qi1Mc, 1.5 Mega pixel) has been attached to the left hand side of the microscope to record bright field (phase) and fluorescent images. Acquisition software used to record images is Nikon NIS elements-F.
Objective lens:
4x LWD Plan-Fluor, NA = 0.13, RI = air, WD = 17.1 mm
10x S Plan Fluor, NA = 0.25, RI =air, WD = 10.5 mm
20x S Plan fluor, NA = 0.45, RI =air, WD = 6.9-8.2 mm
40x S Plan-Apochromat, NA = 0.6, RI = air, WD = 2.8-3.6 mm
40x Superfluor, NA: 1.30, RI = oil, WD =0.22 mm
Turret fluorescence filter set positions:
Position 1: HOECHST/DAPI
Position 2: ECFP
Position 3: EGFP
Position 4: EYFP
Position 5: mCherry
Position 6: blank
The rig is located in room 435 of the ARC building on level 4. Contact person to use the system is Dr John Pediani/email: john.pediani@glasgow.ac.uk.
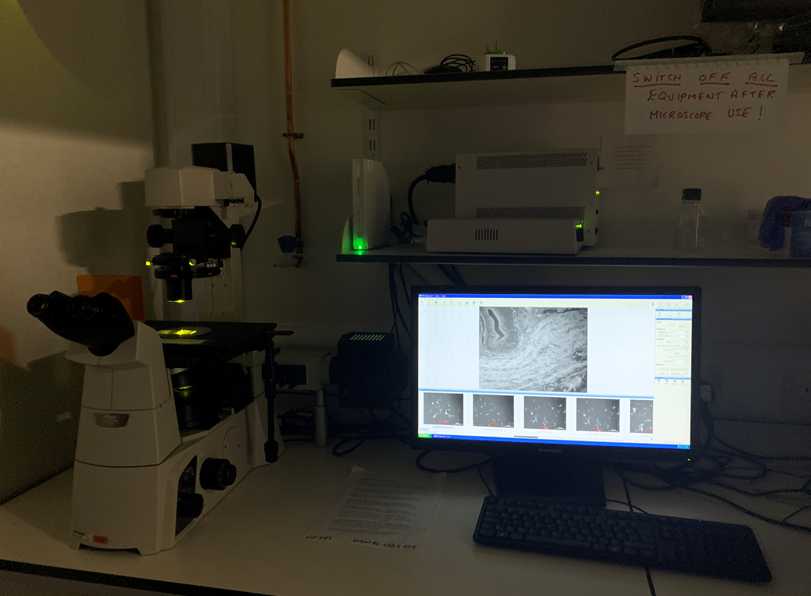
A picture of the Nikon Ti S microscope imaging system is illustrated directly below:
ZEISS AXIOSKOP 2 UPRIGHT EPIFLUORESCENCE RIG WITH ATTACHED AXIOCAM MRM COLOUR CAMERA.
ZEISS AXIOSKOP 2 UPRIGHT EPIFLUORESCENCE RIG WITH ATTACHED AXIOCAM MRM COLOUR CAMERA.
Using a HBO100 Mercury lamp light source, this system has been set up to view the fluorophores DAPI, FITC and TRITC. Attached digital camera permits capture of bright field and fluorescent images.
Objectives lens available:
(Optional) 5x Plan Neofluor (FWD=0.15 mm).
10x/ NA=0.3 Plan Neofluar (FWD=5.2 mm).
20x/NA= 0.5 Plan Neofluar (FWD=2.0 mm).
40x/NA=0.75 Plan Neofluar (oil) DIC M27 (FWD=0.71 mm).
Location:
This upright system is located within room 330 (off main lab Rm 331) of the Davidson building on level 3. An access code is required for door entry and the contact person to use the rig is Shannon Keenan.
ZEISS STEMI 508 DISSECTING MICROSCOPE RIG WITH ATTACHED AXIOCAM 105 COLOUR CAMERA.
ZEISS STEMI 508 DISSECTING MICROSCOPE RIG WITH ATTACHED AXIOCAM 105 COLOUR CAMERA.
This Stereo microscope has been fitted with polarised filters and an Axiocam 105 colour camera (pixel size 2.2 x 2.2 µm) to record birefringent images. It has a 8:1 zoom range with magnifications 2x to 250 possible. Can visualise objects in a field of view up to 122 mm. Adaptor used to couple the camera to the microscope is 0.5x so remember to multiply your final magnification by 0.5 when adding a scale bar to your recorded image.
Location:
Room 329 (off main lab Rm 331) of the Davidson building on level 3. An access code is required to gain entry to the system. Contact person to enquire to book this microscope is Shannon Keenan. A picture of the stereo microscope is depicted below:

LEICA MZ6 DISSECTING MICROSCOPE WITH 470 NM INCIDENT EXCITATION LIGHT FLUORESCENCE MODULE.
LEICA MZ6 DISSECTING MICROSCOPE WITH 470 NM INCIDENT EXCITATION LIGHT FLUORESCENCE MODULE.
System is utilised to localise ‘gross’ FITC/GFP fluorescent signals and the
attached digital camera detector can be used to record bright field and fluorescent images.
Location:
This upright system is located within room 330 (off main lab Rm 331) of the Davidson building on level 3. An access code is required for door entry and contact person to enquire about using or booking this microscope is Shannon Keenan.
ZEISS AXIO OBSERVER A1 EPIFLUORESCENCE (INVERT CONFIGURATION) MICROSCOPE REDOX IMAGING SYSTEM.
ZEISS AXIO OBSERVER A1 EPIFLUORESCENCE (INVERT CONFIGURATION) MICROSCOPE REDOX IMAGING SYSTEM.
This custom built system which has been fitted with 385 and 470 nm LEDs and a long pass emission filter has been set up to perform real-time redox imaging. Acquisition software used to record images is Axiovision 4.8.2.
Location:
This invert system is located within room 444 of the Wolfson link building and the person to contact for training and to book the system is Dr Marcel Van Lith, email Marcel.VanLith@glasgow.ac.uk
MOTORISED LEICA DSM5500 EPIFLUORESCENCE/HISTOLOGY MICROSCOPE.
This motorised epifuorescence microscope has an upright configuration and is located in the Davidson Building within room 207. The rig has been fitted with a monochrome camera (K3M) for fluorescence imaging (2.2 µm pixel size no binning). A colour camera (LEICA K3C) is also available for colour imaging (2.2 µm pixel size no binning). Both cameras have been coupled to the microscope using a 0.7x adaptor.
LASX software is available for automated image acquisition. Following objectives and filter sets are available for imaging your dyes of interest and histology samples:
Filter sets:
DAPI. Ex =360 nm/40 nm BW. Em = 470 nm/40 nm BW.
FITC. Ex = 480 nm/40 nm BW. Em = 527 nm/30 nm BW.
TRITC. Ex = 560 nm/40 nm BW. Em = 645 nm/75 nm BW.
Other filter set is a triple band polychroic filter set for simultaneous detection of DAPI/FITC & TRITC.
Objective Lens:
10x NPlan PH1, (Air), NA = 0.25, WD = 17.7 mm.
20x HCX PL FLUOTAR, (Air), NA = 0.50, WD = 1.27 mm.
40x HCX PL FLUOTAR, (Air), NA = 0.75, WD = 0.4 mm.
100x HCX PL FLUOTAR (Oil), NA = 1.3, WD = 0.18 mm.
To use the rig please email Dr Adam Dobson.
A picture of the microscope is shown below:


