Number of items: 47.
2025
Sun, Youyi, Yang, Zhengxin, Li, Weihao  ORCID: https://orcid.org/0000-0002-0388-7490 and Ganin, Alexey Y.
ORCID: https://orcid.org/0000-0002-0388-7490 and Ganin, Alexey Y.  ORCID: https://orcid.org/0000-0002-3754-5819
(2025)
The investigation of Co₆₋ₓFeₓW₆N (x = 0, 3, 6) as electrocatalysts for the hydrogen evolution reaction.
Dalton Transactions, 54(13),
pp. 5577-5583.
(doi: 10.1039/D4DT03005B)
ORCID: https://orcid.org/0000-0002-3754-5819
(2025)
The investigation of Co₆₋ₓFeₓW₆N (x = 0, 3, 6) as electrocatalysts for the hydrogen evolution reaction.
Dalton Transactions, 54(13),
pp. 5577-5583.
(doi: 10.1039/D4DT03005B)
Qu, Chunlin  ORCID: https://orcid.org/0000-0002-5270-3424, Maini, Isha, Zhang, Jingyi, Ganin, Alexey
ORCID: https://orcid.org/0000-0002-5270-3424, Maini, Isha, Zhang, Jingyi, Ganin, Alexey  ORCID: https://orcid.org/0000-0002-3754-5819 and Moran, David A.J.
ORCID: https://orcid.org/0000-0002-3754-5819 and Moran, David A.J.  ORCID: https://orcid.org/0000-0003-4085-7650
(2025)
Catalytic-enhanced thermal hydrogen-termination of diamond for electronic applications.
Diamond and Related Materials, 154,
112225.
(doi: 10.1016/j.diamond.2025.112225)
ORCID: https://orcid.org/0000-0003-4085-7650
(2025)
Catalytic-enhanced thermal hydrogen-termination of diamond for electronic applications.
Diamond and Related Materials, 154,
112225.
(doi: 10.1016/j.diamond.2025.112225)
2024
Li, W. et al.
(2024)
Reversible K-Ion intercalation in CrSe2 cathodes for potassium-ion batteries: Combined operando PXRD and DFT studies.
Journal of Materials Chemistry A, 12(45),
pp. 31276-31283.
(doi: 10.1039/D4TA05114A)
Rasouli Sadabad, Hamed, Coleman, Heather M., Dooley, James S. G., Snelling, William J., O’Hagan, Barry, Ganin, Alexey Y.  ORCID: https://orcid.org/0000-0002-3754-5819 and Arnscheidt, Joerg
(2024)
Desorption of antibiotics from granular activated carbon during water treatment by adsorption.
Environmental Processes, 11(4),
64.
(doi: 10.1007/s40710-024-00740-4)
ORCID: https://orcid.org/0000-0002-3754-5819 and Arnscheidt, Joerg
(2024)
Desorption of antibiotics from granular activated carbon during water treatment by adsorption.
Environmental Processes, 11(4),
64.
(doi: 10.1007/s40710-024-00740-4)
Wang, Yuanshen, Helmbrech, Katharina, Li, Weihao, Dillenz, Manuel, Wang, Yejun, Groß, Axel and Ganin, Alexey Y.  ORCID: https://orcid.org/0000-0002-3754-5819
(2024)
Overcoming ion trapping in Chevrel Phase compounds via tailored anion substitution: An integrated study of theory, synthesis, and in operando techniques for reversible aqueous Zn-ion batteries.
ACS Applied Materials and Interfaces, 16(38),
pp. 50671-50678.
(doi: 10.1021/acsami.4c09145)
(PMID:39268792)
(PMCID:PMC11440462)
ORCID: https://orcid.org/0000-0002-3754-5819
(2024)
Overcoming ion trapping in Chevrel Phase compounds via tailored anion substitution: An integrated study of theory, synthesis, and in operando techniques for reversible aqueous Zn-ion batteries.
ACS Applied Materials and Interfaces, 16(38),
pp. 50671-50678.
(doi: 10.1021/acsami.4c09145)
(PMID:39268792)
(PMCID:PMC11440462)
Lalaguna, P. L. et al.
(2024)
Spatial control of 2D nanomaterial electronic properties using chiral light beams.
ACS Nano,
(doi: 10.1021/acsnano.4c04506)
(Early Online Publication)
Lau, Elizabeth C.H.T., Cowan, Rhona M., Dodds, Kimberley C., McKenna, Catherine, Ganin, Alexey Y.  ORCID: https://orcid.org/0000-0002-3754-5819, Campopiano, Dominic J. and Yiu, Humphrey H.P.
(2024)
Development of heterogeneous enzymatic cascades with a case study for a separable and recyclable system using a combination of magnetic and non-magnetic supports.
Journal of Chemical Technology and Biotechnology, 99(4),
pp. 759-768.
(doi: 10.1002/jctb.7591)
ORCID: https://orcid.org/0000-0002-3754-5819, Campopiano, Dominic J. and Yiu, Humphrey H.P.
(2024)
Development of heterogeneous enzymatic cascades with a case study for a separable and recyclable system using a combination of magnetic and non-magnetic supports.
Journal of Chemical Technology and Biotechnology, 99(4),
pp. 759-768.
(doi: 10.1002/jctb.7591)
Irshad, Umama Binte, Akhtar, Zareen, Bucher, Götz  ORCID: https://orcid.org/0000-0002-5478-8265, Ganin, Alexey Y.
ORCID: https://orcid.org/0000-0002-5478-8265, Ganin, Alexey Y.  ORCID: https://orcid.org/0000-0002-3754-5819, Siddiqi, Humaira Masood and Schmidt, Bernhard
ORCID: https://orcid.org/0000-0002-3754-5819, Siddiqi, Humaira Masood and Schmidt, Bernhard  ORCID: https://orcid.org/0000-0002-3580-7053
(2024)
Adsorption and photocatalytic properties of Tris(4-aminophenyl)amine-based
polyimide/graphitic carbon nitride composites for organic dye removal.
ACS Applied Polymer Materials, 6(6),
pp. 3390-3401.
(doi: 10.1021/acsapm.4c00019)
ORCID: https://orcid.org/0000-0002-3580-7053
(2024)
Adsorption and photocatalytic properties of Tris(4-aminophenyl)amine-based
polyimide/graphitic carbon nitride composites for organic dye removal.
ACS Applied Polymer Materials, 6(6),
pp. 3390-3401.
(doi: 10.1021/acsapm.4c00019)
Samuel, Arun Kumar, Faqeeh, Abdulhai H., Li, Weihao, Ertekin, Zeliha  ORCID: https://orcid.org/0000-0001-6106-7987, Wang, Yuanshen, Zhang, Jingyi, Gadegaard, Nikolaj
ORCID: https://orcid.org/0000-0001-6106-7987, Wang, Yuanshen, Zhang, Jingyi, Gadegaard, Nikolaj  ORCID: https://orcid.org/0000-0002-3396-846X, Moran, David A.J.
ORCID: https://orcid.org/0000-0002-3396-846X, Moran, David A.J.  ORCID: https://orcid.org/0000-0003-4085-7650, Symes, Mark D.
ORCID: https://orcid.org/0000-0003-4085-7650, Symes, Mark D.  ORCID: https://orcid.org/0000-0001-8067-5240 and Ganin, Alexey Y.
ORCID: https://orcid.org/0000-0001-8067-5240 and Ganin, Alexey Y.  ORCID: https://orcid.org/0000-0002-3754-5819
(2024)
Assessing challenges of 2D-molybdenum ditelluride for efficient hydrogen generation in a full-scale proton exchange membrane (PEM) water electrolyzer.
ACS Sustainable Chemistry and Engineering, 12(3),
pp. 1276-1285.
(doi: 10.1021/acssuschemeng.3c06616)
ORCID: https://orcid.org/0000-0002-3754-5819
(2024)
Assessing challenges of 2D-molybdenum ditelluride for efficient hydrogen generation in a full-scale proton exchange membrane (PEM) water electrolyzer.
ACS Sustainable Chemistry and Engineering, 12(3),
pp. 1276-1285.
(doi: 10.1021/acssuschemeng.3c06616)
2023
Lau, Elizabeth C.H.T., Dodds, Kimberley C., McKenna, Catherine, Cowan, Rhona M., Ganin, Alexey Y.  ORCID: https://orcid.org/0000-0002-3754-5819, Campopiano, Dominic J. and Yiu, Humphrey H.P.
(2023)
Direct purification and immobilization of his-tagged enzymes using unmodified nickel ferrite NiFe2O4 magnetic nanoparticles.
Scientific Reports, 13,
21549.
(doi: 10.1038/s41598-023-48795-x)
(PMID:38057439)
(PMCID:PMC10700653)
ORCID: https://orcid.org/0000-0002-3754-5819, Campopiano, Dominic J. and Yiu, Humphrey H.P.
(2023)
Direct purification and immobilization of his-tagged enzymes using unmodified nickel ferrite NiFe2O4 magnetic nanoparticles.
Scientific Reports, 13,
21549.
(doi: 10.1038/s41598-023-48795-x)
(PMID:38057439)
(PMCID:PMC10700653)
Li, Weihao, Wolff, Niklas, Samuel, Arun Kumar, Wang, Yuanshen, Georgiev, Vihar P.  ORCID: https://orcid.org/0000-0001-6473-2508, Kienle, Lorenz and Ganin, Alexey
ORCID: https://orcid.org/0000-0001-6473-2508, Kienle, Lorenz and Ganin, Alexey  ORCID: https://orcid.org/0000-0002-3754-5819
(2023)
Unlocking high-performance supercapacitor behavior and sustained chemical stability of 2D metallic CrSe2 by optimal electrolyte selection.
ChemElectroChem, 10(21),
e202300428.
(doi: 10.1002/celc.202300428)
ORCID: https://orcid.org/0000-0002-3754-5819
(2023)
Unlocking high-performance supercapacitor behavior and sustained chemical stability of 2D metallic CrSe2 by optimal electrolyte selection.
ChemElectroChem, 10(21),
e202300428.
(doi: 10.1002/celc.202300428)
Mehek, Rimsha, Iqbal, Naseem, Noor, Tayyaba, Wang, Yuanshen and Ganin, Alexey Y.  ORCID: https://orcid.org/0000-0002-3754-5819
(2023)
Efficient electrochemical performance of MnO2 nanowires interknitted vanadium oxide intercalated nanoporous carbon network as cathode for aqueous zinc ion battery.
Journal of Industrial and Engineering Chemistry, 123,
pp. 150-157.
(doi: 10.1016/j.jiec.2023.03.031)
ORCID: https://orcid.org/0000-0002-3754-5819
(2023)
Efficient electrochemical performance of MnO2 nanowires interknitted vanadium oxide intercalated nanoporous carbon network as cathode for aqueous zinc ion battery.
Journal of Industrial and Engineering Chemistry, 123,
pp. 150-157.
(doi: 10.1016/j.jiec.2023.03.031)
Lau, Elizabeth C. H. T., Åhlén, Michelle, Cheung, Ocean, Ganin, Alexey Y.  ORCID: https://orcid.org/0000-0002-3754-5819, Smith, David G.E. and Yiu, Humphrey H. P.
(2023)
Gold-iron oxide (Au/Fe3O4) magnetic nanoparticles as the nanoplatform for binding of bioactive molecules through self-assembly.
Frontiers in Molecular Biosciences, 10,
1143190.
(doi: 10.3389/fmolb.2023.1143190)
(PMID:37051321)
(PMCID:PMC10083301)
ORCID: https://orcid.org/0000-0002-3754-5819, Smith, David G.E. and Yiu, Humphrey H. P.
(2023)
Gold-iron oxide (Au/Fe3O4) magnetic nanoparticles as the nanoplatform for binding of bioactive molecules through self-assembly.
Frontiers in Molecular Biosciences, 10,
1143190.
(doi: 10.3389/fmolb.2023.1143190)
(PMID:37051321)
(PMCID:PMC10083301)
Sun, Youyi, Sviridova, Elizaveta, Kamp, Marius, Zhang, Jingyi, Kienle, Lorenz, Moran, David A.J.  ORCID: https://orcid.org/0000-0003-4085-7650, Guselnikova, Olga and Ganin, Alexey Y.
ORCID: https://orcid.org/0000-0003-4085-7650, Guselnikova, Olga and Ganin, Alexey Y.  ORCID: https://orcid.org/0000-0002-3754-5819
(2023)
Elucidating catalytic sites governing the performance toward the hydrogen evolution reaction in ternary nitride electrocatalysts.
ACS Applied Energy Materials, 6(3),
pp. 1265-1273.
(doi: 10.1021/acsaem.2c02941)
ORCID: https://orcid.org/0000-0002-3754-5819
(2023)
Elucidating catalytic sites governing the performance toward the hydrogen evolution reaction in ternary nitride electrocatalysts.
ACS Applied Energy Materials, 6(3),
pp. 1265-1273.
(doi: 10.1021/acsaem.2c02941)
2022
Fraser, James P. and Ganin, Alexey Y.  ORCID: https://orcid.org/0000-0002-3754-5819
(2022)
The investigation of Mo3Sb7 and Mo3Sb7-xTex as electrocatalysts for the hydrogen evolution reaction.
Results in Chemistry, 4,
100587.
(doi: 10.1016/j.rechem.2022.100587)
ORCID: https://orcid.org/0000-0002-3754-5819
(2022)
The investigation of Mo3Sb7 and Mo3Sb7-xTex as electrocatalysts for the hydrogen evolution reaction.
Results in Chemistry, 4,
100587.
(doi: 10.1016/j.rechem.2022.100587)
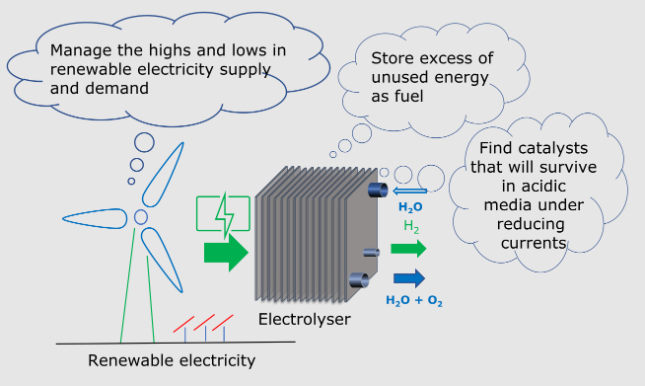
Ganin, Alexey Y.  ORCID: https://orcid.org/0000-0002-3754-5819 and Symes, Mark D.
ORCID: https://orcid.org/0000-0002-3754-5819 and Symes, Mark D.  ORCID: https://orcid.org/0000-0001-8067-5240
(2022)
Towards the application of 2D metal dichalcogenides as hydrogen evolution electrocatalysts in proton exchange membrane electrolyzers.
Current Opinion in Electrochemistry, 34,
101001.
(doi: 10.1016/j.coelec.2022.101001)
ORCID: https://orcid.org/0000-0001-8067-5240
(2022)
Towards the application of 2D metal dichalcogenides as hydrogen evolution electrocatalysts in proton exchange membrane electrolyzers.
Current Opinion in Electrochemistry, 34,
101001.
(doi: 10.1016/j.coelec.2022.101001)
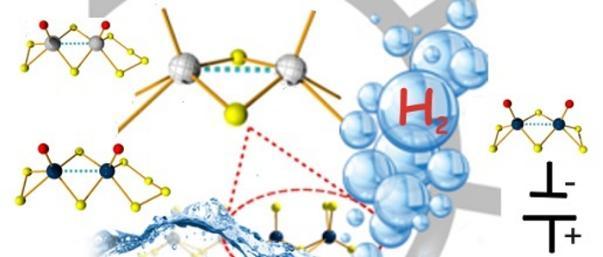
Elliott, Alexander, McAllister, James  ORCID: https://orcid.org/0000-0001-6872-568X, Masaityte, Liudvika, Segado Centellas, Mireia, Long, De-Liang
ORCID: https://orcid.org/0000-0001-6872-568X, Masaityte, Liudvika, Segado Centellas, Mireia, Long, De-Liang  ORCID: https://orcid.org/0000-0003-3241-2379, Ganin, Alexey
ORCID: https://orcid.org/0000-0003-3241-2379, Ganin, Alexey  ORCID: https://orcid.org/0000-0002-3754-5819, Song, Yu-Fei, Bo, Carles and Miras, Haralampos N.
ORCID: https://orcid.org/0000-0002-3754-5819, Song, Yu-Fei, Bo, Carles and Miras, Haralampos N.  ORCID: https://orcid.org/0000-0002-0086-5173
(2022)
Mechanistic insights of molecular metal polyselenides for catalytic hydrogen generation.
Chemical Communications, 58(49),
pp. 6906-6909.
(doi: 10.1039/D2CC01226J)
(PMID:35642784)
ORCID: https://orcid.org/0000-0002-0086-5173
(2022)
Mechanistic insights of molecular metal polyselenides for catalytic hydrogen generation.
Chemical Communications, 58(49),
pp. 6906-6909.
(doi: 10.1039/D2CC01226J)
(PMID:35642784)
Chen, J.-J. et al.
(2022)
Effective storage of electrons in water by the formation of highly reduced polyoxometalate clusters.
Journal of the American Chemical Society, 144(20),
pp. 8951-8960.
(doi: 10.1021/jacs.1c10584)
(PMID:35536652)
Guselnikova, O., Fraser, J.P., Soldatova, N., Sviridova, E., Ivanov, A., Rodriguez, R., Ganin, A.Y.  ORCID: https://orcid.org/0000-0002-3754-5819 and Postnikov, P.
(2022)
The covalent functionalization of few-layered MoTe2 thin films with iodonium salts.
Materials Today Chemistry, 24,
100846.
(doi: 10.1016/j.mtchem.2022.100846)
ORCID: https://orcid.org/0000-0002-3754-5819 and Postnikov, P.
(2022)
The covalent functionalization of few-layered MoTe2 thin films with iodonium salts.
Materials Today Chemistry, 24,
100846.
(doi: 10.1016/j.mtchem.2022.100846)
Sun, Youyi, Wang, Lewen, Guselnikova, Olga, Semyonov, Oleg, Fraser, James, Zhou, Yecheng, López, Núria and Ganin, Alexey Y.  ORCID: https://orcid.org/0000-0002-3754-5819
(2022)
Revealing the activity of Co3Mo3N and Co3Mo3N0.5 as electrocatalysts for the hydrogen evolution reaction.
Journal of Materials Chemistry A, 10(2),
pp. 855-861.
(doi: 10.1039/D1TA08389A)
ORCID: https://orcid.org/0000-0002-3754-5819
(2022)
Revealing the activity of Co3Mo3N and Co3Mo3N0.5 as electrocatalysts for the hydrogen evolution reaction.
Journal of Materials Chemistry A, 10(2),
pp. 855-861.
(doi: 10.1039/D1TA08389A)
2020
Best, Mark G., Cunha-Reis, Cassilda, Ganin, Alexey Y.  ORCID: https://orcid.org/0000-0002-3754-5819, Sousa, Aureliana, Johnston, Jenna, Oliveira, Ana L., Smith, David G. E., Yiu, Humphrey H. P. and Cooper, Ian R.
(2020)
Antimicrobial properties of gallium(III)- and iron(III)-loaded polysaccharides affecting the growth of Escherichia coli, Staphylococcus aureus, and Pseudomonas aeruginosa, in vitro.
ACS Applied Bio Materials, 3(11),
pp. 7589-7597.
(doi: 10.1021/acsabm.0c00811)
ORCID: https://orcid.org/0000-0002-3754-5819, Sousa, Aureliana, Johnston, Jenna, Oliveira, Ana L., Smith, David G. E., Yiu, Humphrey H. P. and Cooper, Ian R.
(2020)
Antimicrobial properties of gallium(III)- and iron(III)-loaded polysaccharides affecting the growth of Escherichia coli, Staphylococcus aureus, and Pseudomonas aeruginosa, in vitro.
ACS Applied Bio Materials, 3(11),
pp. 7589-7597.
(doi: 10.1021/acsabm.0c00811)
Fraser, James P., Postnikov, Pavel, Miliutina, Elena, Kolska, Zdenka, Valiev, Rashid, Švorčík, Vaclav, Lyutakov, Oleksiy, Ganin, Alexey Y.  ORCID: https://orcid.org/0000-0002-3754-5819 and Guselnikova, Olga
(2020)
Application of a 2D molybdenum telluride in SERS-detection of biorelevant molecules.
ACS Applied Materials and Interfaces, 12(42),
pp. 47774-47783.
(doi: 10.1021/acsami.0c11231)
(PMID:32985181)
ORCID: https://orcid.org/0000-0002-3754-5819 and Guselnikova, Olga
(2020)
Application of a 2D molybdenum telluride in SERS-detection of biorelevant molecules.
ACS Applied Materials and Interfaces, 12(42),
pp. 47774-47783.
(doi: 10.1021/acsami.0c11231)
(PMID:32985181)
Sun, Youyi and Ganin, Alexey Y.  ORCID: https://orcid.org/0000-0002-3754-5819
(2020)
The synergistic effects of alloying on the performance and stability of Co3Mo and Co7Mo6 for the electrocatalytic hydrogen evolution reaction.
Hydrogen, 1(1),
pp. 11-21.
(doi: 10.3390/hydrogen1010002)
ORCID: https://orcid.org/0000-0002-3754-5819
(2020)
The synergistic effects of alloying on the performance and stability of Co3Mo and Co7Mo6 for the electrocatalytic hydrogen evolution reaction.
Hydrogen, 1(1),
pp. 11-21.
(doi: 10.3390/hydrogen1010002)
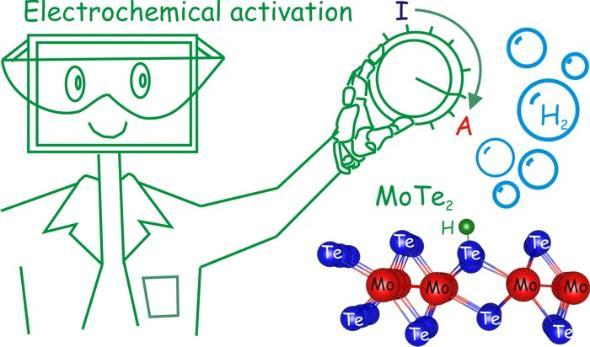
McGlynn, Jessica C., Friskey, Matthew and Ganin, Alexey Y.  ORCID: https://orcid.org/0000-0002-3754-5819
(2020)
Parameter optimisation for electrochemically activated MoTe2.
Sustainable Energy and Fuels, 4(9),
pp. 4473-4477.
(doi: 10.1039/D0SE00684J)
ORCID: https://orcid.org/0000-0002-3754-5819
(2020)
Parameter optimisation for electrochemically activated MoTe2.
Sustainable Energy and Fuels, 4(9),
pp. 4473-4477.
(doi: 10.1039/D0SE00684J)
Fraser, J. P. et al.
(2020)
Selective phase growth and precise-layer control in MoTe2.
Communications Materials, 1,
48.
(doi: 10.1038/s43246-020-00048-4)
Lau, Elizabeth C.H.T., Carvalho, Lucas B., Pereira, Anderson E.S., Montanha, Gabriel S., Corrêa, Camila G., Carvalho, Hudson W.P., Ganin, Alexey Y.  ORCID: https://orcid.org/0000-0002-3754-5819, Fraceto, Leonardo and Yiu, Humphrey H.P.
(2020)
Localization of coated iron oxide (Fe3O4) nanoparticles on tomato seeds and their effects on growth.
ACS Applied Bio Materials, 3(7),
pp. 4109-4117.
(doi: 10.1021/acsabm.0c00216)
ORCID: https://orcid.org/0000-0002-3754-5819, Fraceto, Leonardo and Yiu, Humphrey H.P.
(2020)
Localization of coated iron oxide (Fe3O4) nanoparticles on tomato seeds and their effects on growth.
ACS Applied Bio Materials, 3(7),
pp. 4109-4117.
(doi: 10.1021/acsabm.0c00216)
Tevlek, Atakan, Atya, Abdulraheem M.N., Almemar, Muhannad, Duman, Memed, Gokcen, Dincer, Ganin, Alexey Y.  ORCID: https://orcid.org/0000-0002-3754-5819, Yiu, Humphrey H.P. and Aydin, Halil M.
(2020)
Synthesis of conductive carbon aerogels decorated with β-tricalcium phosphate nanocrystallites.
Scientific Reports, 10,
5758.
(doi: 10.1038/s41598-020-62822-1)
(PMID:32238872)
(PMCID:PMC7113289)
ORCID: https://orcid.org/0000-0002-3754-5819, Yiu, Humphrey H.P. and Aydin, Halil M.
(2020)
Synthesis of conductive carbon aerogels decorated with β-tricalcium phosphate nanocrystallites.
Scientific Reports, 10,
5758.
(doi: 10.1038/s41598-020-62822-1)
(PMID:32238872)
(PMCID:PMC7113289)
Lomax, Bethany A., Conti, Melchiorre, Khan, Nader, Bennett, Nick S., Ganin, Alexey Y.  ORCID: https://orcid.org/0000-0002-3754-5819 and Symes, Mark D.
ORCID: https://orcid.org/0000-0002-3754-5819 and Symes, Mark D.  ORCID: https://orcid.org/0000-0001-8067-5240
(2020)
Proving the viability of an electrochemical process for the simultaneous extraction of oxygen and production of metal alloys from lunar regolith.
Planetary and Space Science, 180,
104748.
(doi: 10.1016/j.pss.2019.104748)
ORCID: https://orcid.org/0000-0001-8067-5240
(2020)
Proving the viability of an electrochemical process for the simultaneous extraction of oxygen and production of metal alloys from lunar regolith.
Planetary and Space Science, 180,
104748.
(doi: 10.1016/j.pss.2019.104748)
2019
McGlynn, J. C. et al.
(2019)
The rapid electrochemical activation of MoTe2 for the hydrogen evolution reaction.
Nature Communications, 10,
4916.
(doi: 10.1038/s41467-019-12831-0)
(PMID:31664018)
(PMCID:PMC6820771)
McAllister, James, Bandeira, Nuno A.G., McGlynn, Jessica C., Ganin, Alexey Y.  ORCID: https://orcid.org/0000-0002-3754-5819, Song, Yu-Fei, Bo, Carles and Miras, Haralampos N.
ORCID: https://orcid.org/0000-0002-3754-5819, Song, Yu-Fei, Bo, Carles and Miras, Haralampos N.  ORCID: https://orcid.org/0000-0002-0086-5173
(2019)
Tuning and mechanistic insights of metal chalcogenide molecular catalysts for the hydrogen-evolution reaction.
Nature Communications, 10,
370.
(doi: 10.1038/s41467-018-08208-4)
(PMID:30670694)
(PMCID:PMC6342911)
ORCID: https://orcid.org/0000-0002-0086-5173
(2019)
Tuning and mechanistic insights of metal chalcogenide molecular catalysts for the hydrogen-evolution reaction.
Nature Communications, 10,
370.
(doi: 10.1038/s41467-018-08208-4)
(PMID:30670694)
(PMCID:PMC6342911)
2018
Koltsov, Iwona, Smalc-Koziorowska, Julita, Prześniak-Welenc, Marta, Małysa, Maria, Kimmel, Giora, McGlynn, Jessica, Ganin, Alexey  ORCID: https://orcid.org/0000-0002-3754-5819 and Stelmakh, Swietlana
(2018)
Mechanism of reduced sintering temperature of Al2O3–ZrO2 nanocomposites obtained by microwave hydrothermal synthesis.
Materials, 11(5),
829.
(doi: 10.3390/ma11050829)
ORCID: https://orcid.org/0000-0002-3754-5819 and Stelmakh, Swietlana
(2018)
Mechanism of reduced sintering temperature of Al2O3–ZrO2 nanocomposites obtained by microwave hydrothermal synthesis.
Materials, 11(5),
829.
(doi: 10.3390/ma11050829)
Crawford, Kevin G.  ORCID: https://orcid.org/0000-0001-8859-930X, Qi, Dongchen, McGlynn, Jessica, Ivanov, Tony G., Shah, Pankaj B., Weil, James, Tallaire, Alexandre, Ganin, Alexey Y.
ORCID: https://orcid.org/0000-0001-8859-930X, Qi, Dongchen, McGlynn, Jessica, Ivanov, Tony G., Shah, Pankaj B., Weil, James, Tallaire, Alexandre, Ganin, Alexey Y.  ORCID: https://orcid.org/0000-0002-3754-5819 and Moran, David A.J.
ORCID: https://orcid.org/0000-0002-3754-5819 and Moran, David A.J.  ORCID: https://orcid.org/0000-0003-4085-7650
(2018)
Thermally stable, high performance transfer doping of diamond using transition metal oxides.
Scientific Reports, 8,
3342.
(doi: 10.1038/s41598-018-21579-4)
(PMID:29463823)
(PMCID:PMC5820251)
ORCID: https://orcid.org/0000-0003-4085-7650
(2018)
Thermally stable, high performance transfer doping of diamond using transition metal oxides.
Scientific Reports, 8,
3342.
(doi: 10.1038/s41598-018-21579-4)
(PMID:29463823)
(PMCID:PMC5820251)
McGlynn, Jessica C., Cascallana-Matías, Irene, Fraser, James P., Roger, Isolda, McAllister, James  ORCID: https://orcid.org/0000-0001-6872-568X, Miras, Haralampos N.
ORCID: https://orcid.org/0000-0001-6872-568X, Miras, Haralampos N.  ORCID: https://orcid.org/0000-0002-0086-5173, Symes, Mark D.
ORCID: https://orcid.org/0000-0002-0086-5173, Symes, Mark D.  ORCID: https://orcid.org/0000-0001-8067-5240 and Ganin, Alexey Y.
ORCID: https://orcid.org/0000-0001-8067-5240 and Ganin, Alexey Y.  ORCID: https://orcid.org/0000-0002-3754-5819
(2018)
Molybdenum ditelluride rendered into an efficient and stable electrocatalyst for the hydrogen evolution reaction by polymorphic control.
Energy Technology, 6(2),
pp. 345-350.
(doi: 10.1002/ente.201700489)
ORCID: https://orcid.org/0000-0002-3754-5819
(2018)
Molybdenum ditelluride rendered into an efficient and stable electrocatalyst for the hydrogen evolution reaction by polymorphic control.
Energy Technology, 6(2),
pp. 345-350.
(doi: 10.1002/ente.201700489)
2017
Romero, F. D. et al.
(2017)
Redox-controlled potassium intercalation into two polyaromatic hydrocarbon solids.
Nature Chemistry, 9(7),
pp. 644-652.
(doi: 10.1038/nchem.2765)
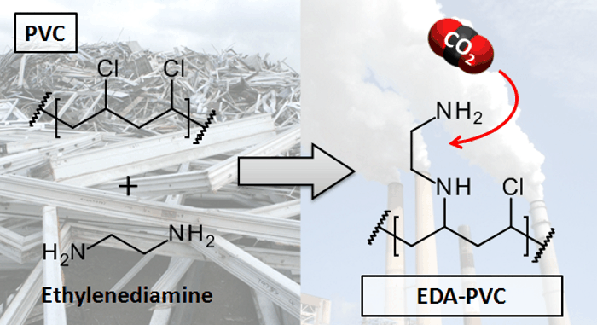
Sneddon, Gregor, McGlynn, Jessica C., Neumann, Marie S., Aydin, Halil M., Yiu, Humphrey H.P. and Ganin, Alexey Y.  ORCID: https://orcid.org/0000-0002-3754-5819
(2017)
Aminated poly(vinyl chloride) solid state adsorbents with hydrophobic function for post-combustion CO2 capture.
Journal of Materials Chemistry A, 5(23),
pp. 11864-11872.
(doi: 10.1039/C7TA00389G)
ORCID: https://orcid.org/0000-0002-3754-5819
(2017)
Aminated poly(vinyl chloride) solid state adsorbents with hydrophobic function for post-combustion CO2 capture.
Journal of Materials Chemistry A, 5(23),
pp. 11864-11872.
(doi: 10.1039/C7TA00389G)
Roger, Isolda, Moca, Roberta, Miras, Haralampos  ORCID: https://orcid.org/0000-0002-0086-5173, Crawford, Kevin G., Moran, David A.J.
ORCID: https://orcid.org/0000-0002-0086-5173, Crawford, Kevin G., Moran, David A.J.  ORCID: https://orcid.org/0000-0003-4085-7650, Ganin, Alexey Y.
ORCID: https://orcid.org/0000-0003-4085-7650, Ganin, Alexey Y.  ORCID: https://orcid.org/0000-0002-3754-5819 and Symes, Mark D.
ORCID: https://orcid.org/0000-0002-3754-5819 and Symes, Mark D.  ORCID: https://orcid.org/0000-0001-8067-5240
(2017)
The direct hydrothermal deposition of cobalt-doped MoS2 onto fluorine-doped SnO2 substrates for catalysis of the electrochemical hydrogen evolution reaction.
Journal of Materials Chemistry A, 5(4),
pp. 1472-1480.
(doi: 10.1039/C6TA08287D)
ORCID: https://orcid.org/0000-0001-8067-5240
(2017)
The direct hydrothermal deposition of cobalt-doped MoS2 onto fluorine-doped SnO2 substrates for catalysis of the electrochemical hydrogen evolution reaction.
Journal of Materials Chemistry A, 5(4),
pp. 1472-1480.
(doi: 10.1039/C6TA08287D)
2015
Sneddon, Gregor, Ganin, Alexey Y.  ORCID: https://orcid.org/0000-0002-3754-5819 and Yiu, Humphrey H.P.
(2015)
Sustainable CO2 adsorbents prepared by coating chitosan onto mesoporous silicas for large-scale carbon capture technology.
Energy Technology, 3(3),
pp. 249-258.
(doi: 10.1002/ente.201402211)
ORCID: https://orcid.org/0000-0002-3754-5819 and Yiu, Humphrey H.P.
(2015)
Sustainable CO2 adsorbents prepared by coating chitosan onto mesoporous silicas for large-scale carbon capture technology.
Energy Technology, 3(3),
pp. 249-258.
(doi: 10.1002/ente.201402211)
Zadik, R. H. et al.
(2015)
Optimized unconventional superconductivity in a molecular Jahn-Teller metal.
Science Advances, 1(3),
e1500059.
(doi: 10.1126/sciadv.1500059)
2014
Kasahara, Y., Takeuchi, Y., Itou, T., Zadik, R.H., Takabayashi, Y., Ganin, A.Y.  ORCID: https://orcid.org/0000-0002-3754-5819, Arčon, D., Rosseinsky, M.J., Prassides, K. and Iwasa, Y.
(2014)
Spin frustration and magnetic ordering in theS=12molecular antiferromagnetfcc−Cs3C60.
Physical Review B, 90,
014413.
(doi: 10.1103/PhysRevB.90.014413)
ORCID: https://orcid.org/0000-0002-3754-5819, Arčon, D., Rosseinsky, M.J., Prassides, K. and Iwasa, Y.
(2014)
Spin frustration and magnetic ordering in theS=12molecular antiferromagnetfcc−Cs3C60.
Physical Review B, 90,
014413.
(doi: 10.1103/PhysRevB.90.014413)
McLennan, Alec G., Ganin, Alexey Y.  ORCID: https://orcid.org/0000-0002-3754-5819, Takabayashi, Yasuhiro, Colman, Ross H., Zadik, Ruth H., Rosseinsky, Matthew J. and Prassides, Kosmas
(2014)
Synthesis of face-centred cubic Cs3C60 in THF.
Faraday Discussions, 173,
pp. 95-103.
(doi: 10.1039/C4FD00085D)
(PMID:25324044)
ORCID: https://orcid.org/0000-0002-3754-5819, Takabayashi, Yasuhiro, Colman, Ross H., Zadik, Ruth H., Rosseinsky, Matthew J. and Prassides, Kosmas
(2014)
Synthesis of face-centred cubic Cs3C60 in THF.
Faraday Discussions, 173,
pp. 95-103.
(doi: 10.1039/C4FD00085D)
(PMID:25324044)
Potocnik, Anton, Krajnc, Andraz, Jeglic, Peter, Takabayashi, Yasuhiro, Ganin, Alexey  ORCID: https://orcid.org/0000-0002-3754-5819, Prassides, Kosmas, Rosseninsky, Matthew J. and Arcon, Denis
(2014)
Size and symmetry of the superconducting gap in the f.c.c. Cs3C60 polymorph close to the metal-Mott insulator boundary.
Scientific Reports, 4,
4265.
(doi: 10.1038/srep04265)
(PMID:24584087)
(PMCID:PMC3939459)
ORCID: https://orcid.org/0000-0002-3754-5819, Prassides, Kosmas, Rosseninsky, Matthew J. and Arcon, Denis
(2014)
Size and symmetry of the superconducting gap in the f.c.c. Cs3C60 polymorph close to the metal-Mott insulator boundary.
Scientific Reports, 4,
4265.
(doi: 10.1038/srep04265)
(PMID:24584087)
(PMCID:PMC3939459)
2013
Potocnick, A., Ganin, A.Y.  ORCID: https://orcid.org/0000-0002-3754-5819, Takabayashi, Y., McDonald, M.T., Heinmaa, I., Jeglic, P., Stern, R., Rosseinsky, M.J., Prassides, K. and Arcon, D.
(2013)
Jahn-Teller orbital glass state in the expanded fcc Cs3C60 fulleride.
Chemical Science, 5(8),
pp. 3008-3017.
(doi: 10.1039/c4sc00670d)
ORCID: https://orcid.org/0000-0002-3754-5819, Takabayashi, Y., McDonald, M.T., Heinmaa, I., Jeglic, P., Stern, R., Rosseinsky, M.J., Prassides, K. and Arcon, D.
(2013)
Jahn-Teller orbital glass state in the expanded fcc Cs3C60 fulleride.
Chemical Science, 5(8),
pp. 3008-3017.
(doi: 10.1039/c4sc00670d)
2011
Bacsa, J., Ganin, A.Y.  ORCID: https://orcid.org/0000-0002-3754-5819, Takabayashi, Y., Christensen, K.E., Prassides, K., Rosseinsky, M.J. and Claridge, J.B.
(2011)
Cation vacancy order in the K0.8+xFe1.6−ySe2 system: five-fold cell expansion accommodates 20% tetrahedral vacancies.
Chemical Science, 2(6),
pp. 1054-1058.
(doi: 10.1039/c1sc00070e)
ORCID: https://orcid.org/0000-0002-3754-5819, Takabayashi, Y., Christensen, K.E., Prassides, K., Rosseinsky, M.J. and Claridge, J.B.
(2011)
Cation vacancy order in the K0.8+xFe1.6−ySe2 system: five-fold cell expansion accommodates 20% tetrahedral vacancies.
Chemical Science, 2(6),
pp. 1054-1058.
(doi: 10.1039/c1sc00070e)
2010
Rabone, J. et al.
(2010)
An adaptable peptide-based porous material.
Science, 329(5995),
pp. 1053-1057.
(doi: 10.1126/science.1190672)
Ganin, A.Y. et al.
(2010)
Polymorphism control of superconductivity and magnetism in Cs3C60 close to the Mott transition.
Nature, 466(7303),
pp. 221-225.
(doi: 10.1038/nature09120)
2009
Takabayashi, Y. et al.
(2009)
The disorder-free non-BCS superconductor Cs3C60 emerges from an antiferromagnetic insulator parent state.
Science, 323(5921),
pp. 1585-1590.
(doi: 10.1126/science.1169163)
2008
Ganin, A.Y.  ORCID: https://orcid.org/0000-0002-3754-5819, Takabayashi, Y., Khimyak, Y.Z., Margadonna, S., Tamai, A., Rosseinsky, M.J. and Prassides, K.
(2008)
Bulk superconductivity at 38 K in a molecular system.
Nature Materials, 7(5),
pp. 367-371.
(doi: 10.1038/nmat2179)
ORCID: https://orcid.org/0000-0002-3754-5819, Takabayashi, Y., Khimyak, Y.Z., Margadonna, S., Tamai, A., Rosseinsky, M.J. and Prassides, K.
(2008)
Bulk superconductivity at 38 K in a molecular system.
Nature Materials, 7(5),
pp. 367-371.
(doi: 10.1038/nmat2179)
This list was generated on Wed Jul 2 21:33:32 2025 BST.