Understanding the biology of CNS leukaemia to develop novel treatments
Figure 1 illustrates potential models for the relationship between bone marrow and CNS leukaemic clones. Our laboratory was the first to provide definitive evidence for model 2. In brief, we established that leukaemic cells freely enter the CNS compartment, without sub-clonal selection, and confirmed that CNS infiltration is highly likely to be present at the time of original diagnosis, even in patients with negative cytology. Therefore, the risk of CNS relapse is determined by the ability to adapt and survive in the CNS niche, rather than by selective entry to the CNS compartment.
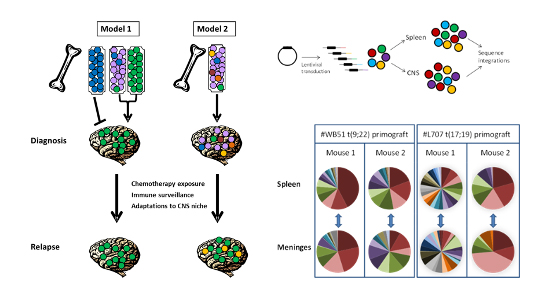
Figure 1: Entry to the CNS is a generic property of ALL blasts: For experimental details see Williams et al Blood 2016;217 p1998-2006
Using biological information to develop novel therapeutics:
Following this discovery, we focussed on identifying factors that enable leukemic blasts to survive in the CNS. We have found several promising targets that may be therapeutically targetable:
Use of MEK-inhibitors to treat ras-mutated CNS-ALL
We identified that the cytokine interleukin-15 provides a growth advantage to ALL blasts under nutrient deprived conditions (such as those found in the CNS)via the ras-raf-MEK-ERK pathway3. We then worked with Prof Julie Irving (Newcastle University) and colleagues to identify a potential role for MEK inhibitors in CNS-ALL (Figure 2)4. This data supported a successful bid to CRUK/Astra Zeneca Combinations Alliance for a Phase I/II trial of MEK-inhibitor Selumetinib plus Dexamethasone in adults and children with relapsed-Ras mutated ALL5.
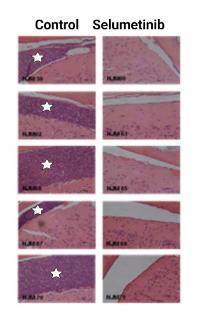
Figure 2: CNS leukaemia is significantly reduced by treatment with the MEK-inhibitor Selumetinib.
Novel Receptor Targets
Working in collaboration with colleagues from Ulm, Germany, we have identified CD79a and IL7Ralpha as novel CNS-ALL drug targets. This has supported preclinical drug development by Biotech companies.
T-Cell ALL
With Dr Frederik van Delft (Newcastle University) we have shown that Dasatinib plus Dexamethasone are highly effective against T-cell CNS-ALL8. Phase I/II trials are in preparation.
Targeting Metabolic vulnerabilities
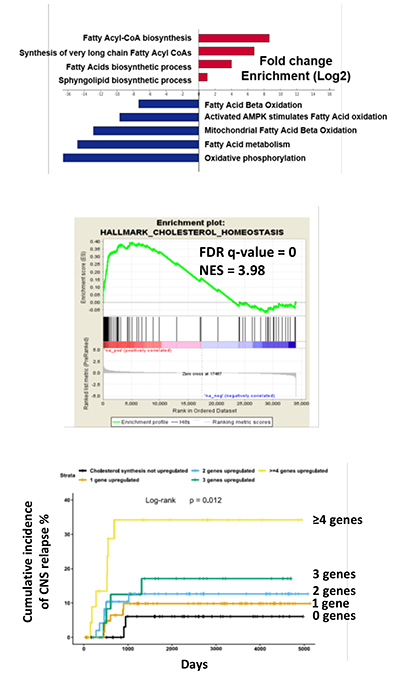
CNS-ALL blasts reside in the leptomeninges, bathed in CSF which is extremely low in nutrients and oxygen. Since leukaemic cells need building blocks and energy to survive and proliferate, and CSF nutrient supplies are scarce, we hypothesized that metabolic remodelling (flexible adaptation of metabolism according to tissue location and environmental conditions) would occur. Work from our laboratory confirmed profound transcriptional adaptation to the CNS niche dominated by metabolic genes highly-enriched for lipid and cholesterol biosynthesis (Figure 3). We have provided proof of concept that stearoyl co-A desaturase (SCD) and cholesterol biosynthesis are clinically relevant targets in CNS-ALL. We are now extending these observations to understand how best to develop novel therapeutics which are less toxic and more effective than our current treatments.
Figure 3: CNS-ALL shows upregulation of lipid and cholesterol biosynthesis, and increased expression of cholesterol synthesis genes is associated with CNS relapse in patients.

