Research Interests
Our group here at Glasgow is interested in the relationship between translating ribosome and nascent polypeptide. Currently this leads us into two main avenues of research.
Membrane protein folding and insertion
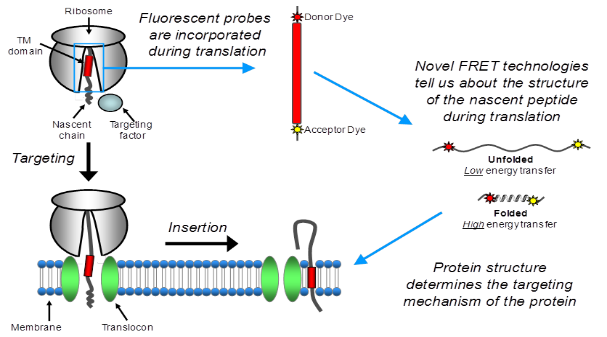
Inner membrane proteins make up 20-30% of the bacterial proteome. The major route of insertion for such proteins is via the Sec machinery. However certain membrane proteins are Sec independent and instead insert via an essential membrane protein called YidC. Despite the importance of YidC, the precise function and mechanism of this protein are difficult to define. In our lab we are using Fluorescence Resonance Energy Transfer (FRET) to monitor structural changes of an inner membrane protein during synthesis on the ribosome, targeting to the YidC pathway and integration into the membrane. This technique enables us to monitor the compaction of α-helices and the formation of tertiary structure, in order to elucidate the timing of folding events that occue between the ribosome exit tunnel and the adoption of the final functional conformation in the membrane. The findings are supported by in vitro insertion assays and crosslinking to the signal recognition (SRP), which provide further details of the role of SRP in YidC targeting.
References:
Robinson, P. J., Findlay J. E. and Woolhead C. A. In Press (2012)
Woolhead, C. A., McCormick P. J. and Johnson A. E. Cell (2004)
Translation Arrest motifs
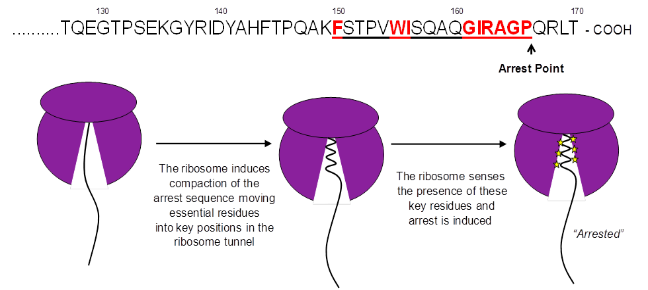
Although ribosomes are responsible for the synthesis of all cellular proteins it was initially believed that translating nascent chains would not interact with the exit tunnel during synthesis. However a small but increasing number of proteins have been identified which interact with the ribosome exit tunnel to induce translational arrest. Escherichia coli secretion monitor (SecM) is one such stalling peptide, SecM monitors the cell export activity through its own translocation to the periplasm and upregulates translation of SecA, an ATPase involved in the SecYEG translocation machinery, when translocation is reduced. How these peptides interact with the exit tunnel is not fully understood however two key features required for stalling include an essential peptide arrest motif and compaction of the nascent chain within the exit tunnel upon stalling. By investigating the SecM sequence found inside the ribosome tunnel and analysis of compaction of the nascent chain in the exit tunnel by structural and biochemical assays we are investigating the interactions between the nascent chain and the exit tunnel which contribute to translation arrest.
References:
Woolhead, C. A. Johnson, A. E. and Bernstein H. D. Molecular Cell (2006)
Work in our laboratory is sponsored by:



