Dr Iain Johnstone
Dr Iain Johnstone
My research is with the genetically tractable model organism Caenorhabditis elegans. Although moderately dissimilar to humans in appearance, at tissue, cellular and molecular levels there are many similarities. Many genes involved in regulatory pathways associated with human disease processes are conserved in C.elegans; well known examples include components of the RAS, Notch, Wnt and insulin signalling pathways, the regulation of apoptosis and genes associated with Alzheimer's and Parkinson's diseases. With a life cycle of only two and a half days, cultured on Petri plates, transgenic strains created in about a week and a wealth of mutant strains available, it is a powerful genetic system to investigate a broad spectrum of processes. Information on the anatomy of the worm is available at Wormatlas, and WormBook is an excellent online resource with sections covering many aspects of worm biology and research. WormBase is the database for C. elegans.
Research interests:
1) Biogenesis of extracellular matrix, focused predominantly on aspects of collagen biology and including homeostasis of the endoplasmic reticulum and quality control of mutant collagens.
2) Tissue-specific aspects of cell cycle control; the interplay between fate specification and function of the central cell cycle regulators.
Collagens and extracellular matrix
 The extracellular matrix (ECM) plays critical roles in the morphogenesis, structure and function of tissues and organs. Without it, organs would be nothing more than a mixed bag of cells. Arguably we currently understand more about the differentiation and development of specific cell types during organ formation, than we do about the regulation of shape and form by the ECM that holds them together. Controlling the interactions between cells and the ECM that specify shape and form will be as critical to our capacity to engineer tissues and organs, as is our capacity to direct stem-cell differentiation.
The extracellular matrix (ECM) plays critical roles in the morphogenesis, structure and function of tissues and organs. Without it, organs would be nothing more than a mixed bag of cells. Arguably we currently understand more about the differentiation and development of specific cell types during organ formation, than we do about the regulation of shape and form by the ECM that holds them together. Controlling the interactions between cells and the ECM that specify shape and form will be as critical to our capacity to engineer tissues and organs, as is our capacity to direct stem-cell differentiation.
Proteins of the collagen superfamily form a diverse range of supramolecular components in the ECM of different tissues. This is reflected in the diverse range of inherited diseases of collagens which include osteogenesis imperfecta, certain sub-types of epidermolysis bullosa and of Ehlers-Danlos syndrome, Alport syndrome, Bethlem myopathy, a variety of chondrodysplasias and some cases of osteoporosis, arterial aneurysms, osteoarthrosis and intervertebral disc disease.
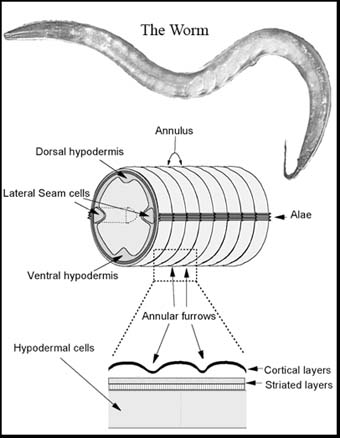
The C.elegans body is effectively an elongate cylinder enclosed within an ECM termed the cuticle (Johnstone, 2000; Page and Johnstone, 2007). This consists predominantly of small collagens encoded by a large gene family. Biogenesis of the cuticle requires the function of various molecular chaperones known to be involved in human collagen biogenesis. Cuticle collagen secretion and assembly is highly regulated and the collagens are subject to highly regulated and extensive crosslinking (Thein et al., 2009). Synthesis is within the underlying epithelial cell-layer (termed the hypodermis); secretion is directional being via the apical surface of the hypodermis and requires COPII secretory vesicle (Roberts et al., 2003) and SM/SNARE functions. The cuticle is patterned by circumferential annular furrows which are created via an interaction with the cytoskeleton in the synthesising hypodermis. Thus, the C. elegans cuticle provides a genetically tractable model for the study of many aspects of collagen and ECM biogenesis.
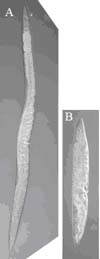 As with some human collagen genes, mutations in C. elegans cuticle collagen genes can affect animal stature. The worm A), right, is wild type and B) is mutant in the dpy-7 cuticle collagen gene. The DPY-7 collagen plays a critical role in the formation and maintenance of the annular furrows, which in turn are necessary for normal body size. This collagen and its partners form specialised collagen sub-structures of narrow bands that locate beneath the annular furrows (McMahon et al., 2003).
As with some human collagen genes, mutations in C. elegans cuticle collagen genes can affect animal stature. The worm A), right, is wild type and B) is mutant in the dpy-7 cuticle collagen gene. The DPY-7 collagen plays a critical role in the formation and maintenance of the annular furrows, which in turn are necessary for normal body size. This collagen and its partners form specialised collagen sub-structures of narrow bands that locate beneath the annular furrows (McMahon et al., 2003).
Immuno-fluorescent localisation of the DPY-7 collagen is shown in A) below. In addition to their stature defect, dpy-7 mutants have a smooth cuticle surface with no annular furrows, indicating that the DPY-7 bands not only locate to the furrows, but are essential for their existence. Therefore this collagen plays a specific role in patterning the surface of this ECM.
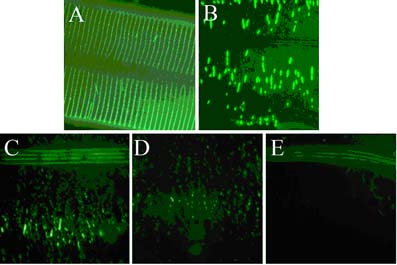 Glycine-substitution mutants are a medically important class of collagen gene mutation. Collagen chains have glycine at every third residue and three monomeric chains assemble into the collagen triple helix. The glycine residues face into the centre of the triple helix. Substitution of glycine with any other amino acid disrupts the triple helix and in humans such lesions are frequently dominant. For this reason, mutations in collagen genes are the most frequent spontaneous heritable disease-causing mutants of humans. The images in C), D), and E) are immuno-localisation of a glycine-substitution mutant version of the DPY-7 collagen, cultured at 15oC, 20oC and 25oC respectively. Varying amounts of a highly fragmented structure are assembled in a temperature sensitive way. We are using cuticle collagen glycine substitution mutants to investigate the behaviour and processing of this medically important class of mutant collagen. The image in B) shows a partial suppressor mutant that causes more glycine substitution mutant DPY-7 collagen to be successfully secreted and assembled.
Glycine-substitution mutants are a medically important class of collagen gene mutation. Collagen chains have glycine at every third residue and three monomeric chains assemble into the collagen triple helix. The glycine residues face into the centre of the triple helix. Substitution of glycine with any other amino acid disrupts the triple helix and in humans such lesions are frequently dominant. For this reason, mutations in collagen genes are the most frequent spontaneous heritable disease-causing mutants of humans. The images in C), D), and E) are immuno-localisation of a glycine-substitution mutant version of the DPY-7 collagen, cultured at 15oC, 20oC and 25oC respectively. Varying amounts of a highly fragmented structure are assembled in a temperature sensitive way. We are using cuticle collagen glycine substitution mutants to investigate the behaviour and processing of this medically important class of mutant collagen. The image in B) shows a partial suppressor mutant that causes more glycine substitution mutant DPY-7 collagen to be successfully secreted and assembled.
Tissue-specific cell cycle control
The C. elegans intestine consists of precisely 20 cells that are organised as a tube. To create the tube, intestinal cells are shaped like half a donut.
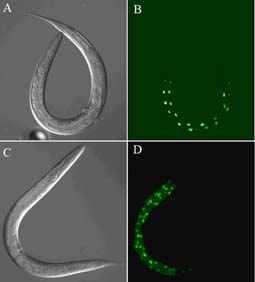 A) and B) are of a wild type C. elegans; the GFP expression in B) is driven by a intestinal-specific transgene and located to nuclei with a nuclear localization signal. C) and D) are of a gain-of-function mutant in the C. elegans gene cdc-25.1 (Clucas et al., 2002). This mutation causes an intestinal hyperplasia, as indicated by the GFP expression. Although the CDC-25.1 protein is required positively to drive the cell cycle in many or all cells of the early C. elegans embryo, its deregulation by the gain-of-function mutation only results in the intestinal hyperplasia.
A) and B) are of a wild type C. elegans; the GFP expression in B) is driven by a intestinal-specific transgene and located to nuclei with a nuclear localization signal. C) and D) are of a gain-of-function mutant in the C. elegans gene cdc-25.1 (Clucas et al., 2002). This mutation causes an intestinal hyperplasia, as indicated by the GFP expression. Although the CDC-25.1 protein is required positively to drive the cell cycle in many or all cells of the early C. elegans embryo, its deregulation by the gain-of-function mutation only results in the intestinal hyperplasia.
We have investigated the nature of the gain-of-function mutation. CDC-25.1 is supplied maternally to the embryo, as is the F-box protein LIN-23. LIN-23 controls a progressive decay of CDC-25.1 in all blastomeres of the early embryo; the gain-of-function cdc-25.1 mutant escapes this negative regulation (Segref et al.). However, this does not fully explain the tissue specific nature of the hyperplasia.
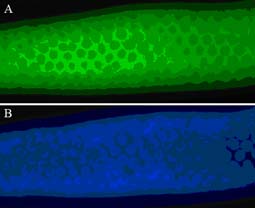 The LIN-23 protein is present in the developing C. elegans germline A), right, where it surrounds the syncytial germline nuclei seen by DAPI staining B). It appears to be largely excluded from the germline nuclei. During germline development the LIN-23 protein is loaded into developing oocytes where after fertilisation it initiates the regulated decay of CDC-25.1. Key questions include how LIN-23 function itself is regulated and what other targets does it have in the embryo.
The LIN-23 protein is present in the developing C. elegans germline A), right, where it surrounds the syncytial germline nuclei seen by DAPI staining B). It appears to be largely excluded from the germline nuclei. During germline development the LIN-23 protein is loaded into developing oocytes where after fertilisation it initiates the regulated decay of CDC-25.1. Key questions include how LIN-23 function itself is regulated and what other targets does it have in the embryo.
Selected References
Clucas, C., Cabello, J., Bussing, I., Schnabel, R., and Johnstone, I.L. (2002). Oncogenic potential of a C. elegans cdc-25 gene is demonstrated by a gain-of-function allele. EMBO J 21, 665-674.
Johnstone, I.L. (2000). Cuticle collagen genes. Expression in Caenorhabditis elegans. Trends Genet 16, 21-27.
McMahon, L., Muriel, J.M., Roberts, B., Quinn, M., and Johnstone, I.L. (2003). Two sets of interacting collagens form functionally distinct substructures within a Caenorhabditis elegans extracellular matrix. Mol Biol Cell 14, 1366-1378.
Page, A.P., and Johnstone, I.L. (2007). The cuticle. WormBook, 1-15.
Roberts, B., Clucas, C., and Johnstone, I.L. (2003). Loss of SEC-23 in Caenorhabditis elegans causes defects in oogenesis, morphogenesis, and extracellular matrix secretion. Mol Biol Cell 14, 4414-4426.
Segref, A., Cabello, J., Clucas, C., Schnabel, R., and Johnstone, I.L. Fate Specification and Tissue-specific Cell Cycle Control of the C. elegans Intestine. (2010). Mol Biol Cell.
Thein, M.C., Winter, A.D., Stepek, G., McCormack, G., Stapleton, G., Johnstone, I.L., and Page, A.P. (2009). The combined extracellular matrix cross-linking activity of the peroxidase MLT-7 and the duox BLI-3 are critical for post-embryonic viability in Caenorhabditis elegans. J Biol Chem.

