Professor Andy Waters
- Professor, Dean of Internationalisation (Parasitology)
telephone:
01413308720
email:
Andy.Waters@glasgow.ac.uk
Room B413, Sir Graeme Davies Building, 120 University Place, Glasgow, G12 8TA
Research interests
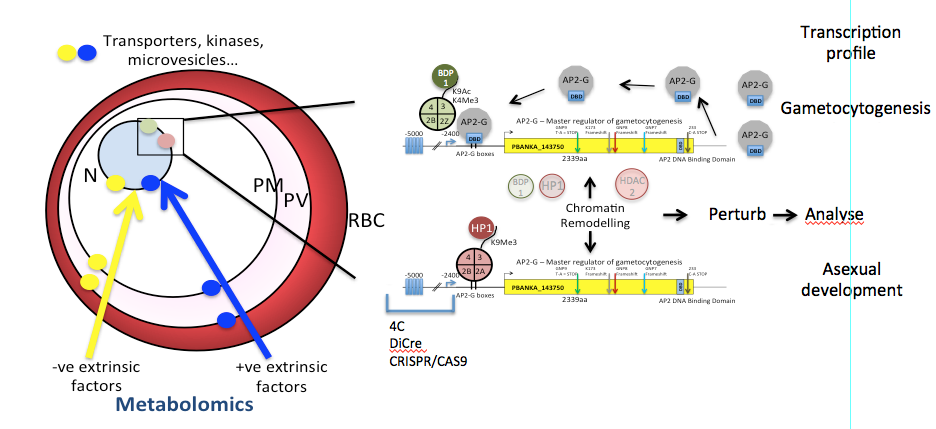
Prof Waters has >30 years experience in malaria research and together with Dr Chris Janse helped pioneer the development of the rodent model of malaria research, Plasmodium berghei in Leiden. He is a Wellcome Trust Principal Research Fellow with current funding until 2021. The major objective of the Glasgow laboratory is to understand the molecular developmental biology that is associated with sexual development.
There are three main themes to the research:
1. Triggers for gametocytogenesis. A transcription factor underpinning commitment to this critical event has been recently identified in the laboratory (Sinha et al (2014) Nature). Its mode of action and control of its expression are currently under study.
2. Influence of host environment on parasite development. This is an interest largely driven by metabolomics and seeks to understand the resources available to the intracellular parasite and the consequences of those resources for drug development and parasite maturation.
3. Development of tools for the more sophisticated genetic engineering of malaria parasites
The laboratory analyses these issues using a polyomics approach which is institutionalised in Glasgow and includes: metabolomics, NGS, proteomics, parasite genetic manipulation and complemented by a fully functioning insectary. Technology development is also a key part of our activities where we have recently developed three new approaches to inducible regulation of gene/protein expression with a view to assigning function to genes/proteins
Our laboratory currently has four post-doctoral researchers with a great range of molecular biological expertise, four technical support staff and four PhD students. Our activities are complemented by three other research groups working on malaria or Toxoplasma and through our participation in the WTCMP of which Prof Waters is Director. Together with the many other parasitological groups at Glasgow there is the greatest concentration and breadth of molecular parasitology expertise in the UK. Prof. Waters was also Scientific Director of Evimalar, a European Commission-funded Network of Excellence that improved European and global collaboration in the basic research of malaria parasite biology and their interactions with their hosts and vectors. Evimalar has now devolved to form Evimalar EEIG (European Economic Interest Group) which contains 13 founder members and can partner traditional institutions in bids for international funding.
Our Group:
PPDRAs and Fellows
Dr Nisha Philip has extensive experience in signalling and miRNAs in Plasmodium and has recently developed an inducible protein degradation systems (Philip & Waters, 2015)
Dr Katie Hughes has extensive experience in Plasmodium cell biology and protozoan molecular biology.
Dr Brett Roberts has just joined the laboratory and will focus on parasite genetic manipulation.
Dr Sebastian Kirchner is a Marie Curie International Fellow studying epigenetic regulation of Plasmodium gene expression.
PhD students: thesis titles.
Ms Rebecca Lee: “The Natural History of Plasmodium Gametocytes”
Ms Robyn Kent: “Experimentally Inducible Gametocytogenesis in Plasmodium”
Ms Joanne Power; “ Epigenetics and Gametocytogenesis in Plasmodium”
There is currently a vacancy for an experienced bioinformatician.
The Tech Team
Ms Anne Graham is the Laboratory Manager and responsible for the co-ordination of the laboratory activities. She has extensive experience in molecular biology.
Ms Heli Vaikinnen has extensive experience in the molecular biology of protozoan parasites.
Mrs Angela McBride runs the group’s insectary facility Mr John Archer has expertise in informatics and parasitology
Updated: 4.11.2015
Grants
Grants and Awards listed are those received whilst working with the University of Glasgow.
- Permission to proliferate: identifying regulators of blood stage schizogony in malaria parasites
Medical Research Council
2024 - 2029
- AFRIBOP 2024: Supporting international collaborations and training with African partners.
Scottish Funding Council
2023 - 2024
- Unravelling mechanisms of stage conversion in malaria parasites
Wellcome Trust
2023 - 2028
- Initiative to Develop African Research Leaders [IDeAL]
Science for Africa Foundation
2023 - 2027
- Comparative analysis of commitment to gametocytogenesis and sex determination in human and rodent-infectious malaria parasites
Wellcome Trust
2023 - 2027
- In-situ transcriptomic and proteomic Subcellular molecular Imager
Medical Research Council
2022 - 2023
- Iron usage in the eukaryotic parasite Toxoplasma gondii
Wellcome Trust
2019 - 2024
- Blantyre-Blantyre Clinical Research Project
Scottish Government
2017 - 2022
- Stage differentiation in malaria parasites
The Royal Society
2016 - 2021
- Gene expression in Plasmodium parasites: the molecular mechanics of gametocytogenesis (and variant transcription of genes)
Wellcome Trust
2016 - 2021
- SECOMAP
European Commission
2015 - 2017
- Identification and analysis of essential proteins involved in Toxoplasma gondii mitochondrial tRNA import (ISSF Fellowship)
Wellcome Trust
2015 - 2015
- Biology and Pathology of the Malaria Parasite
The Company of Biologists
2014 - 2015
- Role of sub-activation-threshold TCR interactions in maintaining T cell memory
National Institutes of Health
2014 - 2016
- The Wellcome Centre for Molecular Parasitology ( Core Support )
Wellcome Trust
2014 - 2021
- The Wellcome Centre for Molecular Parasitology (Core support)
Wellcome Trust
2014 - 2021
- Identification and analysis of essential proteins involved in Toxoplasma gondii mitochondrial biogenesis (ISSF Catalyst)
Wellcome Trust
2014 - 2014
- Wellcome Trust Liverpool-Glasgow Centre for Global Health Research
Wellcome Trust
2013 - 2018
- Institutional Strategic Support Fund (ISSF)
Wellcome Trust
2012 - 2018
- ParaMet - A systematic approach to understanding parasite metabolism.
European Commission
2012 - 2016
- Ozmalnet⌒TOWARDS THE ESTABLISHMENT OF A FRAMEWORK IN MALARIA BETWEEN THE AMRN AND EVIMALAR
European Commission
2012 - 2014
- OzmalnetTOWARDS THE ESTABLISHMENT OF A FRAMEWORK IN MALARIA BETWEEN THE AMRN AND EVIMALAR
European Commission
2012 - 2014
- Integrated Health - Polyomics and Systems Biomedicine (ISSF Bid)
Wellcome Trust
2011 - 2014
- Redefining roles of epigenetic regulators: ALBAs in translational control
European Molecular Biology Organization
2010 - 2012
- Towards the establishment of a permanent European Virtual Institutededicated to Malaria Research (EVIMalaR)
European Commission
2009 - 2015
- Conditional translational repression: a core regulatory mechanism of gene expression during development of the malaria parasite.
Wellcome Trust
2008 - 2015
- Search for optimised inhibitors of Plasmodium falciparum
Medicines for Malaria Venture
2008 - 2008
Additional information
Editorial Board
- 2005 - present: Future Microbiology - Member Editorial Advisory Board
- 2001 - present: Molecular & Biochemical Parasitology - Executive Editor
- 2000 - 2005: Molecular & Biochemical Parasitology - Member Editorial Board & Reviews Editor
- 1998 - 2008: Trends in Parasitology - Member Editorial Advisory Board
Grant Advisory Board
- 2013 - present: Center of Infection and Immunity of Lille, France - Committee member for external evaluation of Center of Infection and Immunity of Lille for Agence d'Evaluation de la Recherche et des etablissements d'Enseignement Superieur (AERES)
- 2013 - 2017: Wellcome Trust Sanger Institute - Malaria Programme Scientific Advisory Board Member
- 2013 - present: Institute Cochin, Paris, France - Committee member for external evaluation of Department of Infection, Immunology & Inflammation for Agence d'Evaluation de la Recherche et des etablissements d'Enseignement Superieur (AERES)
- 2012 - present: Glasgow, Scotland, UK - Co-Organiser, Glasgow Polyomics International Symposium
- 2012 - present: German Research Foundation (DFG), Bonn, Germany - Invited Expert Reviewer by DFG in the field of Immunology, Inflammation & Infection Biology
- 2012 - present: Wellcome Trust/Royal Society - Member of the Interview Panel for the Wellcome Trust/Royal Society Sir Henry Dale Fellowships
- 2011 - present: NIH Bethesda, Maryland, USA - Invited expert reviewer of proposals received in response to RFP-NIAID-DMID-NIH-AI2010100 entitled: "An Integrated Approach to Understanding Host-Pathogens Interactions"
- 2010 - present: Wellcome Trust Expert Group - Member, Malaria 1990 to date: key influences & the role of the Wellcome Trust
- 2009 - 2014: Biomalpar - Director Biomalpar, a European Community-funded Network of Excellence
- 2007: WHO/TDR - Consultant to Steering Committee on Pathogenesis and Applied Genomics
- 2007: International Livestock Research Institute (ILRI) - Consultant on development of anti-parasitic diagnostics and vaccines in the post-genomic era
- 2007: Wellcome Trust - Member External review Committee for PhD Programme
- 2006 - 2008: WHO/TDR - PLUS Review panel member for parasite applied genomics
- 2006 - 2008: Nederlandse Organisatie voor Wetenschappelijke Onderzoek (NWO) - Rubicon award, review panel member
- 2006: French Medical Research Council (INSERM) - Appointed INSERM expert peer reviewer
- 2006 - 2009: European ANTIMAL Network - Member of External Scientific Advisory Committee
- 2005 - 2010: WHO - Member Parasite Transfection Task Force and Drug Resistance
- 2004 - 2009: European Commission Framework VI - Member Executive Scientific Steering Committee and Scientific Cluster Co-ordinator, Network of Excellence, Biology of Malaria Parasites (BIOMALPAR)
- 2002 - 2005: The Malaria Research and Reference Reagent Resource Center (MR4) - Member Scientific Advisory Panel, MR4, ATCC, Manassas, VA, USA
- 2001: Institut Pasteur - Member, External Evaluation Committee, Department of Immunology
- 2001 - 2004: Wellcome Trust - Member, Infection & Immunity Panel
- 2001 - 2007: Nederlandse Organisatie voor Wetenschappelijke Onderzoek (NWO) - Member, Microbiology Grants Panel, Aard-en Levenswetenschappen (ALW)
Invited International Presentations
- 2014: Heidelberg, Germany - Invited Speaker, Biomalpar 10, The Annual Evimalar Meeting, EMBL
- 2014: Hinxton, England, UK - Invited Speaker and Tutor, Advanced Course on Parasite Genetics, Wellcome Trust Sanger Institute
- 2014: Nijmegen, The Netherlands - Invited Speaker, Department of Molecular Biology, Radboud University Medical Centre
- 2013: Berkhamsted, England, UK - Invited Speaker, 2nd Wellcome Trust Researcher Meeting
- 2013: San Feliu de Guixols, Spain - Invited Speaker: Comparative Genomics of Eukaryotic Microorganisms: Patterns of Complexity in Eukaryotic Genomes
- 2013: Lucca, Italy - Invited Speaker, Gordon Conference on Malaria
- 2013: Snowmass, Colorado, USA - Invited Speaker, FASEB Summer Research Conference "Microbial Pathogenesis: Mechanisms of Infectious Disease"
- 2013: New Orleans, Louisiana (USA) - Co-organiser and speaker, Keystone meeting, Malaria
- 2012: Lille, France - Invited Speaker, Molecular Parasitology Symposium, Institute Pasteur
- 2012: London, England, UK - Invited Speaker, Inaugural Wellcome Trust Researcher Meeting
- 2012: Glasgow, Scotland, UK - Co-organiser & speaker, Introduction to Metabolomics, an Evimalar Training Course
- 2012: Warwick, England, UK - Symposium Organiser, "Molecular Motors" SGM Autumn Meeting
- 2012: Hinxton, England, UK - Invited speaker and tutor, Advanced Course on Parasite Genetics, Wellcome Trust Sanger Institute
- 2012: Lorne, Victoria, Australia - Invited Speaker and member of International Scientific Organising Committee, "Molecular Approaches to Malaria" Conference
- 2012: Santa Fe, New Mexico, USA - Invited plenary speaker, Joint Keystone Symposia on Drug Discovery for Protozoan Parasites/Fungal Pathogens: From Basic Biology to Drug Discovery
- 2011: Philadelphia, Pennsylvania, USA - Invited Speaker, Department of Microbiology, University of Pennsylvania School of Medicine
- 2011: Guaruja, Brazil - Member of the International Scientific Committee, Symposium Chair and Speaker, of the 1st International Meeting on Cell Biology of Pathogens (IMCBP) joint with American Society of Cell Biology (ASCB)
- 2011: Bethesda, Maryland, USA - Invited Speaker, "Gametocyte Biology", Sponsored by MIH and Gates Foundation
- 2010: Kampala, Uganda - Invited lecturer, Genes & Genomes in the Tropics, an Evimalar sponsored course, University of Kampala
- 2010: Edinburgh, Scotland, UK - Invited Speaker, "Parasite to prevention: Advances in the understanding of malaria, a Biomed Central Conference
- 2010: Heidelberg, Germany - Invited Speaker, EMBO Fellows Inaugural Conference
- 2010: Melbourne, Australia - Symposium Chair (x2) & Invited Speaker (x3), ICOPA XII
- 2010: BPRC - Invited Speaker, Laudatio for Dr Alan Thomas
- 2010: Heidelberg, Germany - Organiser 6th Biomalpar conference
- 2010: Edinburgh, Scotland, UK - Symposium co-organiser and chair (with Dr. M. Ginger), Society for General Microbiology AGM
- 2010: Hinxton, England, UK - Invited Speaker, Wellcome Trust Sanger Centre
- 2009: Woods Hole, Massachusetts, USA - Invited Speaker, Biology of Parasitism
- 2009: Aberdeen, Scotland, UK - Invited Speaker, University of Aberdeen, Department of Medical Microbiology
- 2009: Oxford, England, UK - Invited Speaker, Malaria Gordon Conference
- 2009: Baltimore, Maryland, USA - Invited Speaker, 7th Malaria Conference of the Malaria Institute, Johns Hopkins University
- 2008: Lorne, Australia - Invited Speaker, 3rd MAM Conference
- 2008: Singapore - Invited Speaker, 2nd Malaria Conference, Nangyang TU
- 2008: Melbourne, Australia - Invited Speaker, Genome Technology Access Centre (GTAC), presentation made to an invited audience of High School students
- 2008: Woods Hole, Massachusetts, USA - Invited Speaker, Biology of Parasitism
- 2008: Brussels, Belgium - White paper author and rapporteur at "Challenges for the Future: Research on HIV/AIDS, Malaria and Tuberculosis" FP7 policy conference for FP8
- 2008: Alpbach, Austria - Invited Speaker Keystone Symposium on Malaria: Immunology, Pathogenesis and Vaccine Perspectives
- 2008: Rhode Island, USA - Invited Speaker, Gordon Research Conference on the Biology of Host-Parasite Interactions
- 2007: San Feliu de Guixols, Spain - Invited Speaker, 2nd ESF-EMBO Symposium Comparative Genomics of Eukaryotic Microorganisms: "Eukaryotic Genome Evolution from Yeasts to Parasites and Pathogens"
- 2007: Leiden, The Netherlands - Invited speaker, Centre of Infectious Diseases symposium, Leiden University Medical Centre
- 2007: Woods Hole, Massachusetts, USA - Invited Speaker, Biology of Parasitism Course, MBL
- 2007: Bern, Switzerland - Invited Speaker, University of Bern Graduate School Cell Biology
- 2007: Arnhem, The Netherlands - Invited Speaker, Dutch Microbiology Society Annual Meeting
- 2007: Bangkok, Thailand - Course Faculty, Malaria Parasite Transfection Workshop
- 2007: Siena, Italy - Invited Speaker 1st Global Novartis Infectious Disease Meeting
- 2007: St. Louis, Missouri, USA - Invited Speaker, Department of Microbiology, Washington University
- 2007: Salvador, Bahia, Brazil - Invited Speaker, XXXVI Annual Meeting of the Brazilian Society of Biochemistry and Molecular Biology (SBBq)
- 2006: Puerto Rico - Invited Speaker, University of Puerto Rico
- 2006: Glasgow, Scotland, UK - Invited Speaker, University of Glasgow, Division of Life Sciences
- 2006: Woods Hole, Massachusetts, USA - Invited Speaker, Biology of Parasitism Course, MBL
- 2006: Woods Hole, Massachusetts, USA - Co-organiser, Molecular Parasitology Meeting
- 2006: Jerusalem, Israel - Invited Speaker, workshop on Severe Malaria, Israeli Society for Parasitology, Protozoology and Tropical Diseases (ISPPTD)
- 2006: Dresden, Germany - Speaker, COST 857 Conference
- 2006: Rhode Island, USA - Invited Speaker, Gordon Research Conference on the Biology of Host-Parasite Interactions
- 2006: Glasgow, Scotland, UK - Invited Speaker, University of Glasgow, Division of Life Sciences
- 2006: Matsuyama, Japan - Invited Speaker, Matsuyama International Symposium on Cell-Free Sciences
- 2006: Leiden, The Netherlands - Invited Speaker, Federation of Biomedical Scientific Societies (FMWV-NWO)
- 2006: Atlanta, Georgia, USA - Invited Speaker, "Knockouts (and knock-ins) in parasites: promises and challenges" pre-meeting workshop, American Society of Tropical Medicine and Hygiene (ASTMH) meeting
- 2006: Heidleberg, Germany - Lead organiser, EU FP6 NoE BIOMALPAR Annual Meeting
- 2006: Singapore - External Examiner, Thesis Jaysree Iyer, University of Nan-Yang
- 2006: Dundee, Scotland, UK - Invited Speaker, University of Dundee
- 2006: Taos, New Mexico, USA - Invited Speaker, Keystone Symposium on Malaria: Functional Genomics to Biology to Medicine
- 2005: New Delhi, India - Course Faculty, Malaria Parasite Transfection Workshop
- 2005: Veyrier du Lac, France - Invited Panelist, "Future of Anti-Parasitic Vaccines" meeting, Merieux Foundation
- 2005: Woods Hole, Massachusetts, USA - Invited Speaker, Biology of Parasitism course
- 2005: San Feliu de Guixols, Spain - Invited Speaker ESF-EMBO Conference, Comparative genomics of eukaryotic microorganisms: eukaryotic genome evolution, approaches with yeasts and fungi
- 2005: Oxford, England, UK - Organiser and Chair, Gordon Research Conference on Malaria
- 2005: Boston, Massachusetts, USA - Invited participant, "Future of Malaria Genomics", Policy forum sponsored by Burroughs Wellcome Fund and Wellcome Trust
- 2005: Woods Hole, Massachusetts, USA - Co-organiser, Molecular Parasitology Meeting
- 2005: Washington, DC, USA - Invited Speaker, Plasmodium pre-erythrocytic development; genomic/proteomic approaches to intervention
- 2004: Paris, France - Invited Speaker at "Dynamic Imaging Microscopy & Analysis for Biologists" Workshop, Institut Pasteur
- 2004: Lorne, Australia - Speaker & Session Chair, MAM04
- 2004: Chester, England, UK - Invited Keynote Speaker, British Society for Parasitology, Annual meeting
- 2004: Les Treilles, France - Invited participant in New Frontiers in Malaria Parasite and Anopheles Mosquito Engineering, Fondation des Treilles
- 2004: Woods Hole, Massachusetts, USA - Invited Speaker, Biology of Parasitism course
- 2004: Bangkok, Thailand - Invited Speaker and Discussant, WHO Workshop, "The impact of genetic engineering of Plasmodium on parasite drug resistance"
- 2004: Miami, Florida, USA - Invited Speaker, Symposium on "Transmission Blocking Vaccines", ASTMH Meeting
- 2004: London, England, UK - Invited Speaker, Keynote Lecture Series, NIMR, Mill Hill
- 2004: Singapore - Invited Speaker, Molecular Approaches to Malaria, Nanyang Technological University, Singapore
- 2004: Baltimore, Maryland, USA - Invited Speaker, 2nd Malaria Conference of the Malaria Institute, Johns Hopkins University
- 2004: Melbourne, Australia - Invited speaker at Walter and Elisa Hall Institute
- 2004: Woods Hole, Massachusetts, USA - Co-organiser, Molecular Parasitology Meeting
- 2003: Aubiere, France - Plenary Speaker, Bienniale Reunion Protozologists
- 2003: Amsterdam, The Netherlands - Symposium speaker, Phacilitate Vaccine development Congress
- 2003: Kindrogan, Scotland, UK - British Society for Parasitology Kindrogan Speaker, SUMP
- 2003: Edinburgh, Scotland, UK - Invited speaker, University of Edinburgh
- 2003: Heidelberg, Germany - Invited speaker, ZDF Spearpoint programme, University of Heidelberg
- 2003: Paris, France - Plenary Speaker, European Conference on Computational Biology 2003
- 2003: Glasgow, Scotland, UK - Invited Speaker, Wellcome Institute for Parasitology Lecture Series, University of Glasgow
- 2003: Manchester, England - Symposium speaker, "Post Genomics Applied to Processes: Advances in Eukaryotic Microbiology", Society for General Microbiology
- 2003: Oxford, England, UK - Vice chairman, Gordon Research Conference on Malaria
- 2003: Utrecht, The Netherlands - Invited Speaker, Dutch Microbiological Society, de Uithof
- 2003: Odense, Denmark - Invited Speaker, University of Southern Denmark
- 2002: Tucson, Arizona, USA - Invited speaker, Vector Pathogen interactions meeting
- 2002: Woods Hole, Massachusetts, USA - Session Chair, Molecular Parasitology Meeting
- 2002: Woods Hole, Massachusetts, USA - Invited speaker, Molecular Parasitology Course
- 2002: Kindrogan, Scotland, UK - Plenary speaker, SUMP
- 2002: Stockholm, Sweden - Invited Speaker, Pathology of Malaria, Meeting, Karolinska Institute
- 2002: Umea, Sweden - Speaker, external lecture series, University of Umea
- 2001: Keele, England, UK - Plenary Speaker, British Society for Parasitology
- 2001: London, England, UK - Invited Speaker, "Functional Genomics of Protozoan Parasites", Royal Society of Great Britain
- 2001: Hinxton, England, UK - Invited participant, 11th Malaria Genome Meeting, Wellcome Trust
- 2001: Woods Hole, Massachusetts, USA - Invited speaker, Molecular Parasitology Course
- 2001: Oxford, England, UK - Invited speaker, Gordon Malaria Conference
Prizes, Awards and Distinctions
- 2010: Royal Society of Edinburgh - Elected Fellow
- 2009: European Virtual Institute for Malaria Research (Evimalar) - Director, Evimalar
- 2009: European Molecular Biology Organisation - Elected member
- 2008: Wellcome Trust - Principal Research Fellow
- 2006: Edinburgh, Scotland, UK - Visiting Professor, University of Edinburgh
- 2003: London, England, UK - Honorary Reader, Imperial College
- 2001: Oeiras, Portugal - Visiting Fellow, Gulbenkian Institute of Science
Professional Learned Society
- 2010 - present: Royal Society of Edinburgh - Fellow
- 2009 - present: European Molecular Biology Organisation - Elected Member
- 2009 - present: Society for General Microbiology
- 2000 - 2008: British Society for Parasitology
- 1998 - 2007: Dutch Parasitological Society (NVP)
- 1996 - 2001: Royal Society of Tropical Medicine and Hygiene
Research Fellowship
- 2008 - 2015: Wellcome Trust Principal Research Fellowship



